Page 22 - JCTR-9-5
P. 22
324 Mahmood et al. | Journal of Clinical and Translational Research 2023; 9(5): 322-326
Table 1. Patient and tumor characteristics Table 2. Liver function parameters
Total patients, N (%) 55 (100) Baseline (range) Post‑TARE P
Age at diagnosis, years (range)
Median 60 Alkaline phosphatase (U/mL) 112.0 (36.0–782.0) 198 (73–1442.0) <0.001
Range 36–84 Albumin (g/dL) 4 (1.8–4.9) 3.55 (2.2–4.7) <0.004
Gender, N (%) Bilirubin (mg/dL) 0.5 (0.2–2.3) 0.8 (0.2–5.6) <0.001
Male 21 (38.2) ALT (U/mL) 24 (8–149) 28.5 (9–173) <0.004
Female 34 (61.8) AST (U/mL) 28.5 (13–100) 39.5 (12–121) <0.001
Ethnicity, N (%) Abbreviations: ALT: Alanine transaminase; AST: Aspartate transaminase
Caucasian 30 (54.5)
Hispanic 9 (16.4) A
Asian 9 (16.4)
Other 7 (12.7)
ECOG performance status, N (%)
0 18 (32.7)
1 32 (58.2)
2 5 (9.1)
Tumor sidedness, N (%)
Left 40 (72.7)
Right 13 (23.6)
Unknown 2 (3.6) B
Primary tumor resected, N (%)
Yes 50 (90.9)
No 5 (9.1)
TARE, N (%)
Unilobar 13 (23.6)
Bilobar 42 (76.4)
Re-treatment 8 (14.5)
MSI-high, N (%)
Yes 2 (3.6)
No 32 (58.2)
Unknown 21 (38.2)
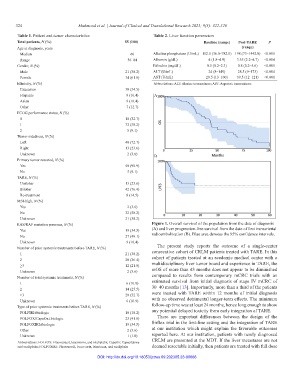
RAS/RAF mutation presence, N (%) Figure 1. Overall survival of the population from the date of diagnosis
Yes 19 (34.5) (A) and liver progression-free survival from the date of first transarterial
No 27 (49.1) radioembolization (B). Blue area denotes the 95% confidence intervals.
Unknown 9 (16.4)
Number of prior systemic treatments before TARE, N (%) The present study reports the outcome of a single-center
1 21 (38.2) consecutive cohort of CRLM patients treated with TARE. In this
2 20 (36.4) cohort of patients treated at an academic medical center with a
≥3 12 (21.8) multidisciplinary liver tumor board and experience in TARE, the
Unknown 2 (3.6) mOS of more than 43 months does not appear to be diminished
Number of total systemic treatments, N (%) compared to results from contemporary mCRC trials with an
1 6 (10.9) estimated survival from initial diagnosis of stage IV mCRC of
2 14 (25.5) 30–40 months [13]. Importantly, more than a third of the patients
≥3 29 (52.7) were treated with TARE within 12 months of initial diagnosis
Unknown 6 (10.9) with no observed detrimental longer-term effects. The minimum
Type of prior systemic treatments before TARE, N (%) follow-up time was at least 24 months, hence long enough to show
FOLFIRI±biologic 10 (18.2) any potential delayed toxicity from early integration of TARE.
FOLFOX/CapeOx±biologic 23 (41.8) There are important differences between the design of the
FOLFOXIRI±biologic 19 (34.5) Sirflox trial in the first-line setting and the integration of TARE
Other 2 (3.6) at our institution which might explain the favorable outcomes
Unknown 1 (1.8) reported here. At our institution, patients with newly diagnosed
Abbreviations: FOLFOX: Fluorouracil, leucovorin, and oxaliplatin; CapeOx: Capecitabine CRLM are presented at the MDT. If the liver metastases are not
and oxaliplatin; FOLFOXIRI: Fluorouracil, leucovorin, irinotecan, and oxaliplatin deemed resectable initially, then patients are treated with full dose
DOI: http://dx.doi.org/10.18053/jctres.09.202305.23-00066