Page 10 - JCTR-9-6
P. 10
374 Sempere-Rubio et al. | Journal of Clinical and Translational Research 2023; 9(6): 369-380
Table 2. Quality assessment scores (AMSTAR)
Study 1 2 3 4 5 6 7 8 9 10 11 12 13 Score
Antunes et al. [19] 2 1 0 1 1 2 2 2 2 0 0 1 0 14
Elizagaray-García et al. [20] 2 2 0 2 1 2 2 2 2 0 0 2 1 18
García-Ríos et al. [21] 2 2 0 2 1 2 2 2 2 0 0 1 1 17
Saracoglu et al. [22] 2 2 2 2 1 2 2 2 2 2 2 2 0 23
Suso-Martí et al. [23] 1 2 2 0 2 2 1 2 2 2 2 2 2 22
Notes: 1. Explicitly described to allow replication; 2. Adequate number and range of databases; 3. Alternative searches; 4. Adequate range of key words; 5. Non-English-language papers included
in the search; 6. Inclusion criteria explicitly described to allow replication; 7. Excludes reviews which do not adequately address inclusion and exclusion criteria; 8. Two independent reviewers
assessing selection bias; 9. Quality assessment explicitly described to allow replication; 10. Meta-analysis conducted on only homogeneous data or limitations to homogeneity discussed;
11. Confidence intervals/effect sizes reported where possible; 12. Conclusions supported by the meta-analysis or other data analysis findings; 13. Conclusions address levels of evidence for each
intervention/comparison
Table 3. Summary of findings and quality of evidence (PAGAC)
2018 PAGAC Magnitude and Overall
Systematic review Applicability Generalizability Risk of bias or Quantity and precision of effect grade
research questions study limitations consistency
Pain intensity Strong Limited Limited Limited Not assignable Limited
Quality of life Strong Limited Limited Limited Not assignable Limited
Functionality Moderate Limited Limited Limited Not assignable Limited
Anxiety Moderate Limited Limited Limited Not assignable Limited
Pain catastrophizing Moderate Limited Limited Limited Not assignable Limited
PAGAC: Physical activity guidelines advisory committee grading criteria
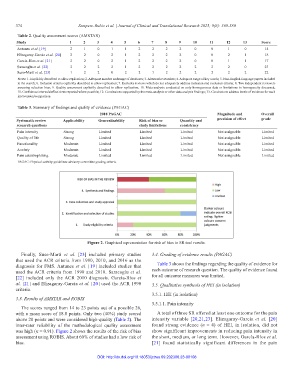
Figure 2. Graphical representation for risk of bias in SR tool results.
Finally, Suso-Martí et al. [23] included primary studies 3.4. Grading of evidence results (PAGAC)
that used the ACR criteria from 1990, 2010, and 2016 as the
diagnosis for FMS. Antunes et al. [19] included studies that Table 3 shows the findings regarding the quality of evidence for
used the ACR criteria from 1990 and 2010. Saracoglu et al. each outcome of research question. The quality of evidence found
[22] included only the ACR 2010 diagnosis. García-Ríos et for all outcome measures was limited.
al. [21] and Elizagaray-García et al. [20] used the ACR 1990 3.5. Qualitative synthesis of HEI (in isolation)
criteria.
3.5.1. HEI (in isolation)
3.3. Results of AMSTAR and ROBIS
3.5.1.1. Pain intensity
The scores ranged from 14 to 23 points out of a possible 26,
with a mean score of 18.8 points. Only two (40%) study scored A total of three SR offered at least one outcome for the pain
above 20 points and were considered high-quality (Table 2). The intensity variable [20,21,23]. Elizagaray-García et al. [20]
inter-rater reliability of the methodological quality assessment found strong evidence (n = 4) of HEI, in isolation, did not
was high (κ = 0.91). Figure 2 shows the results of the risk of bias show significant improvements in reducing pain intensity in
assessment using ROBIS. About 60% of studies had a low risk of the short, medium, or long term. However, García-Ríos et al.
bias. [21] found statistically significant differences in the pain
DOI: http://dx.doi.org/10.18053/jctres.09.202306.23-00108