Page 57 - MI-2-4
P. 57
Microbes & Immunity Management of obesity
Microaerophillic Functions
Stomach
pH 1–2
<10 CFU/mL
2
Digestion and acid
secretion
Small Intestine Digestion and absorption
pH 6–7 of carbohydrates,
10 1–9 CFU/mL proteins, and fats
Absorption of vitamin B12
and bile acid salts
Large Intestine
pH 5–7
10 10–12 CFU/mL Absorption of water,
Anaerobes electrolytes,and short
chain fatty acids
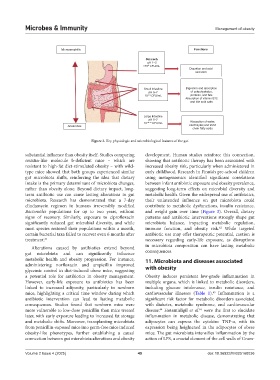
Figure 2. Key physiologic and microbiological features of the gut
substantial influence than obesity itself. Studies comparing development. Human studies reinforce this connection,
resistin-like molecule b-deficient mice – which are showing that antibiotic therapy has been associated with
resistant to high-fat diet-stimulated obesity – with wild- increased obesity risk, particularly when administered in
type mice showed that both groups experienced similar early childhood. Research in Finnish pre-school children
gut microbiota shifts, reinforcing the idea that dietary using metagenomics identified significant correlations
intake is the primary determinant of microbiota changes, between infant antibiotic exposure and obesity prevalence,
rather than obesity alone. Beyond dietary impact, long- suggesting long-term effects on microbial diversity and
term antibiotic use can cause lasting alterations in gut metabolic health. Given the widespread use of antibiotics,
microbiota. Research has demonstrated that a 7-day their unintended influence on gut microbiota could
clindamycin regimen in humans irreversibly modified contribute to metabolic dysfunctions, insulin resistance,
Bacteroides populations for up to two years, without and weight gain over time (Figure 3). Overall, dietary
signs of recovery. Similarly, exposure to ciprofloxacin patterns and antibiotic interventions strongly shape gut
significantly reduced gut microbial diversity, and while microbiota balance, impacting metabolic regulation,
most species restored their populations within a month, immune function, and obesity risk. While targeted
62
certain bacterial taxa failed to recover even 6 months after antibiotic use may offer therapeutic potential, caution is
treatment. 61 necessary regarding early-life exposure, as disruptions
Alterations caused by antibiotics extend beyond in microbiota composition can have lasting metabolic
gut microbiota and can significantly influence consequences.
metabolic health and obesity progression. For instance, 11. Microbiota and diseases associated
administering norfloxacin and ampicillin improved with obesity
glycemic control in diet-induced obese mice, suggesting
a potential role for antibiotics in obesity management. Obesity induces persistent low-grade inflammation in
However, early-life exposure to antibiotics has been multiple organs, which is linked to metabolic disorders,
linked to increased adiposity, particularly in newborn including glucose intolerance, insulin resistance, and
mice, highlighting a critical time window during which cardiovascular illnesses (Table 1). Inflammation is a
63
antibiotic intervention can lead to lasting metabolic significant risk factor for metabolic disorders associated
consequences. Studies found that newborn mice were with diabetes, metabolic syndrome, and cardiovascular
64
65
more vulnerable to low-dose penicillin than mice treated disease. Hotamisligil et al. were the first to elucidate
later, with early exposure leading to increased fat storage inflammation in metabolic disease, demonstrating that
and metabolic shifts. Moreover, transplanting microbiota adipocytes can express the cytokine TNF-α, with its
from penicillin-exposed mice into germ-free mice induced expression being heightened in the adipocytes of obese
obesity-like phenotypes, further establishing a causal mice. The gut microbiota intensifies inflammation by the
connection between gut microbiota alterations and obesity action of LPS, a crucial element of the cell walls of Gram-
Volume 2 Issue 4 (2025) 49 doi: 10.36922/MI025160036