Page 11 - MSAM-4-2
P. 11
Materials Science in Additive Manufacturing Hydrogels in mandibular reconstruction
A
B
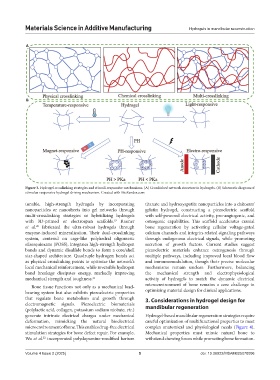
Figure 3. Hydrogel crosslinking strategies and stimuli-responsive mechanisms. (A) Crosslinked network structure in hydrogels. (B) Schematic diagram of
stimulus-responsive hydrogel driving mechanism. Created with BioRender.com
tunable, high-strength hydrogels by incorporating titanate and hydroxyapatite nanoparticles into a chitosan/
nanoparticles or nanosheets into gel networks through gelatin hydrogel, constructing a piezoelectric scaffold
multi-crosslinking strategies or hybridizing hydrogels with self-powered electrical activity, pro-angiogenic, and
19
with 3D-printed or electrospun scaffolds. Rauner osteogenic capabilities. This scaffold accelerates cranial
et al. fabricated the ultra-robust hydrogels through bone regeneration by activating cellular voltage-gated
20
enzyme-induced mineralization. Their dual-crosslinking calcium channels and integrin-related signaling pathways
system, centered on cage-like polyhedral oligomeric through endogenous electrical signals, while promoting
silsesquioxane (POSS), integrates high-strength hydrogen secretion of growth factors. Current studies suggest
bonds and dynamic disulfide bonds to form a core/shell piezoelectric materials enhance osteogenesis through
star-shaped architecture. Quadruple hydrogen bonds act multiple pathways, including improved local blood flow
as physical crosslinking points to optimize the network’s and immunomodulation, though their precise molecular
local mechanical reinforcement, while reversible hydrogen mechanisms remain unclear. Furthermore, balancing
bond breakage dissipates energy, markedly improving the mechanical strength and electrophysiological
mechanical strength and toughness. 21 activity of hydrogels to match the dynamic electrical
Bone tissue functions not only as a mechanical load- microenvironment of bone remains a core challenge in
bearing system but also exhibits piezoelectric properties optimizing material design for clinical applications.
that regulate bone metabolism and growth through 3. Considerations in hydrogel design for
electromagnetic signals. Piezoelectric biomaterials
(polylactic acid, collagen, potassium sodium niobate, etc.) mandibular regeneration
generate intrinsic electrical charges under mechanical Hydrogel-based mandibular regeneration strategies require
deformation, mimicking the natural bioelectrical careful optimization of multifunctional properties to meet
microenvironment of bone. This enables drug-free electrical complex anatomical and physiological needs (Figure 4).
stimulation strategies for bone defect repair. For example, Mechanical properties must mimic natural bone to
Wu et al. incorporated polydopamine-modified barium withstand chewing forces while promoting bone formation.
22
Volume 4 Issue 2 (2025) 5 doi: 10.36922/MSAM025070006