Page 29 - TD-3-3
P. 29
Tumor Discovery Adjuvant immunotherapy in high-risk melanoma
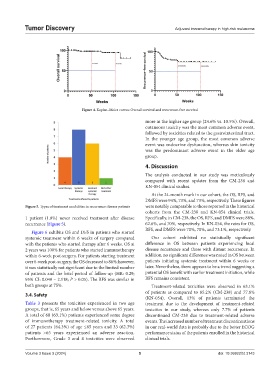
Figure 4. Kaplan–Meier curves: Overall survival and recurrence-free survival
more in the higher age group (24.6% vs. 10.5%). Overall,
cutaneous toxicity was the most common adverse event,
followed by toxicities related to the gastrointestinal tract.
In the younger age group, the most common adverse
event was endocrine dysfunction, whereas skin toxicity
was the predominant adverse event in the older age
group.
4. Discussion
The analysis conducted in our study was meticulously
compared with recent updates from the CM-238 and
KN-054 clinical studies.
At the 24-month mark in our cohort, the OS, RFS, and
DMFS were 94%, 73%, and 73%, respectively. These figures
Figure 5. Types of treatment modalities in recurrence disease patients were notably comparable to those reported in the historical
cohorts from the CM-238 and KN-054 clinical trials.
1 patient (1.0%) never received treatment after disease Specifically, in CM-238, the OS, RFS, and DMFS were 88%,
recurrence (Figure 5). 62.6%, and 70%, respectively. In KN-054, the rates for OS,
RFS, and DMFS were 70%, 70%, and 73.1%, respectively.
Figure 6 exhibits OS and DFS in patients who started
systemic treatment within 6 weeks of surgery compared Our cohort exhibited no statistically significant
with the patients who started therapy after 6 weeks. OS at difference in OS between patients experiencing local
2 years was 100% for patients who started immunotherapy disease recurrence and those with distant recurrence. In
within 6-week post-surgery. For patients starting treatment addition, no significant difference was noted in OS between
over 6-week post-surgery, the OS decreased to 86%; however, patients initiating systemic treatment within 6 weeks or
it was statistically not significant due to the limited number later. Nevertheless, there appears to be a trend suggesting a
of patients and the brief period of follow-up (HR: 0.29; potential OS benefit with earlier treatment initiation, while
95% CI: 0.040 – 2.118; P > 0.05). The RFS was similar in RFS remains consistent.
both groups at 75%. Treatment-related toxicities were observed in 63.1%
of patients as compared to 85.2% (CM-238) and 77.8%
3.4. Safety
(KN-054). Overall, 13% of patients terminated the
Table 3 presents the toxicities experienced in two age treatment due to the development of treatment-related
groups, that is, 65 years and below versus above 65 years. toxicities in our study, whereas only 7.7% of patients
A total of 60 (63.1%) patients experienced some degree discontinued CM-238 due to treatment-related adverse
of immunotherapy treatment-related toxicity. A total events. The increased number of treatment discontinuations
of 27 patients (64.3%) of age ≤65 years and 33 (62.3%) in our real-world data is probably due to the better ECOG
patients >65 years experienced an adverse reaction. performance status of the patients enrolled in the historical
Furthermore, Grade 3 and 4 toxicities were observed clinical trials.
Volume 3 Issue 3 (2024) 5 doi: 10.36922/td.3143