Page 30 - TD-3-3
P. 30
Tumor Discovery Adjuvant immunotherapy in high-risk melanoma
Figure 6. Kaplan–Meier curves: Overall survival and recurrence-free survival in immunotherapy started within 6 weeks versus after 6 weeks
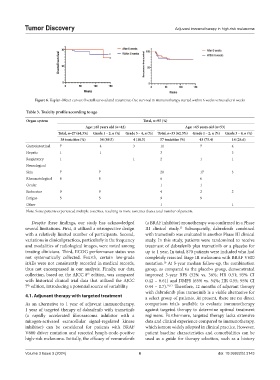
Table 3. Toxicity profile according to age
Organ system Total, n=95 (%)
Age: ≤65 years old (n=42) Age: >65 years old (n=53)
Total, n=27 (64.3%) Grade 1 – 2, n (%) Grade 3 – 4, n (%) Total, n=33 (62.3%) Grade 1 – 2, n (%) Grade 3 – 4, n (%)
38 toxicities (%) 34 (89.5) 4 (10.5) 57 toxicities (%) 43 (75.4) 14 (24.6)
Gastrointestinal 7 4 3 11 7 4
Hepatic 1 1 3 3
Respiratory 1 1 2 1 1
Neurological
Skin 7 7 20 17 3
Rheumatological 8 8 6 6
Ocular 1 1
Endocrine 9 9 4 2 2
Fatigue 5 5 9 9
Other 1 1
Note: Some patients experienced multiple toxicities, resulting in more toxicities than a total number of patients.
Despite these findings, our study has acknowledged (a BRAF inhibitor) monotherapy was confirmed in a Phase
several limitations. First, it utilized a retrospective design III clinical study. Subsequently, dabrafenib combined
15
with a relatively limited number of participants. Second, with trametinib was evaluated in another Phase III clinical
variations in clinical practices, particularly in the frequency study. In this study, patients were randomized to receive
and modalities of radiological images, were noted among treatment of dabrafenib plus trametinib or a placebo for
treating clinicians. Third, ECOG performance status was up to 1 year. In total, 870 patients were included who had
not systematically collected. Fourth, certain low-grade completely resected Stage III melanoma with BRAF V600
irAEs were not consistently recorded in medical records, mutation. At 5-year median follow-up, the combination
16
thus not encompassed in our analysis. Finally, our data group, as compared to the placebo group, demonstrated
collection, based on the AJCC 8 edition, was compared improved 5-year RFS (52% vs. 36%; HR 0.51; 95% CI
th
with historical clinical trial data that utilized the AJCC 0.42 – 0.61) and DMFS (65% vs. 54%; HR 0.55; 95% CI
7 edition, introducing a potential source of variability. 0.44 – 0.7). 16,17 Therefore, 12 months of adjuvant therapy
th
with dabrafenib plus trametinib is a viable alternative for
4.1. Adjuvant therapy with targeted treatment a select group of patients. At present, there are no direct
As an alternative to 1 year of adjuvant immunotherapy, comparison trials available to evaluate immunotherapy
1 year of targeted therapy of dabrafenib with trametinib against targeted therapy to determine optimal treatment
(a rapidly accelerated fibrosarcoma inhibitor with a regimens. Furthermore, targeted therapy lacks extensive
mitogen-activated extracellular signal-regulated kinase data and clinical experience compared to immunotherapy,
inhibitor) can be considered for patients with BRAF which is more widely adopted in clinical practice. However,
V600 driver mutation and resected lymph-node-positive patient baseline characteristics and comorbidities can be
high-risk melanoma. Initially, the efficacy of vemurafenib used as a guide for therapy selection, such as a history
Volume 3 Issue 3 (2024) 6 doi: 10.36922/td.3143