Page 12 - TD-4-3
P. 12
Tumor Discovery FBXW7 in Leukemia
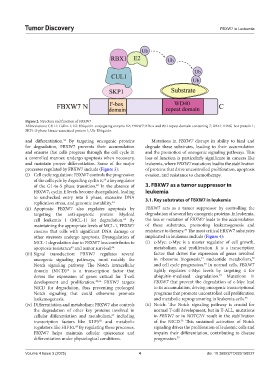
Figure 2. Structure and function of FBXW7
Abbreviations: CUL1: Cullin 1; E2: Ubiquitin-conjugating enzyme E2; FBXW7: F-box and WD repeat domain-containing 7; RBX1: RING-box protein 1;
SKP1: S-phase kinase-associated protein 1; Ub: Ubiquitin.
and differentiation. By targeting oncogenic proteins Mutations in FBXW7 disrupt its ability to bind and
58
for degradation, FBXW7 prevents their accumulation degrade these substrates, leading to their accumulation
and ensures that cells progress through the cell cycle in and the promotion of oncogenic signaling pathways. This
a controlled manner, undergo apoptosis when necessary, loss of function is particularly significant in cancers like
and maintain proper differentiation. Some of the major leukemia, where FBXW7 mutations lead to the stabilization
processes regulated by FBXW7 include (Figure 3): of proteins that drive uncontrolled proliferation, apoptosis
(i) Cell cycle regulation: FBXW7 controls the progression evasion, and resistance to chemotherapy.
of the cell cycle by degrading cyclin E, a key regulator
59
of the G1-to-S phase transition. In the absence of 3. FBXW7 as a tumor suppressor in
60
FBXW7, cyclin E levels become dysregulated, leading leukemia
to unchecked entry into S phase, excessive DNA 3.1. Key substrates of FBXW7 in leukemia
replication stress, and genomic instability. 61
(ii) Apoptosis: FBXW7 also regulates apoptosis by FBXW7 acts as a tumor suppressor by controlling the
targeting the anti-apoptotic protein Myeloid degradation of several key oncogenic proteins. In leukemia,
cell leukemia 1 (MCL-1) for degradation. By the loss or mutation of FBXW7 leads to the accumulation
62
maintaining the appropriate levels of MCL-1, FBXW7 of these substrates, promoting leukemogenesis and
70
ensures that cells with significant DNA damage or resistance to therapy. The most critical FBXW7 substrates
other stressors undergo apoptosis. Dysregulation of implicated in leukemia include (Figure 4):
MCL-1 degradation due to FBXW7 loss contributes to (i) c-Myc: c-Myc is a master regulator of cell growth,
apoptosis resistance and tumor survival. 64 metabolism, and proliferation. It is a transcription
63
(iii) Signal transduction: FBXW7 regulates several factor that drives the expression of genes involved
71
72
oncogenic signaling pathways, most notably the in ribosome biogenesis, nucleotide metabolism,
73
Notch signaling pathway. The Notch intracellular and cell cycle progression. In normal cells, FBXW7
domain (NICD) is a transcription factor that tightly regulates c-Myc levels by targeting it for
65
74
drives the expression of genes critical for T-cell ubiquitin-mediated degradation. Mutations in
development and proliferation. 40,66 FBXW7 targets FBXW7 that prevent the degradation of c-Myc lead
NICD for degradation, thus preventing prolonged to its accumulation, driving oncogenic transcriptional
Notch signaling that could otherwise promote programs that promote uncontrolled cell proliferation
leukemogenesis. and metabolic reprogramming in leukemia cells. 75
(iv) Differentiation and metabolism: FBXW7 also controls (ii) Notch: The Notch signaling pathway is crucial for
the degradation of other key proteins involved in normal T-cell development, but in T-ALL, mutations
cellular differentiation and metabolism, including in FBXW7 or in NOTCH1 result in the stabilization
67
transcription factors like KLF5 and metabolic of the NICD. This sustained activation of Notch
76
68
regulators like HIF1α. By regulating these processes, signaling drives the proliferation of leukemic cells and
69
FBXW7 helps maintain cellular quiescence and impairs their differentiation, contributing to disease
differentiation under physiological conditions. progression. 77
Volume 4 Issue 3 (2025) 4 doi: 10.36922/TD025150027