Page 14 - AJWEP-22-5
P. 14
Rajak, et al.
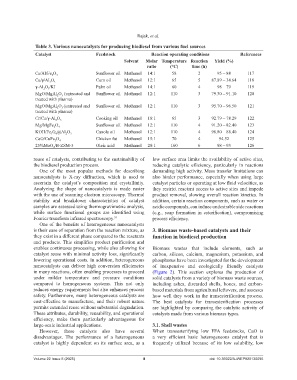
Table 3. Various nanocatalysts for producing biodiesel from various fuel sources
Catalyst Feedstock Reaction operating conditions References
Solvent Molar Temperature Reaction Yield (%)
ratio (°C) time (h)
Cs/Al/Fe O 4 Sunflower oil Methanol 14:1 58 2 95 – 88 117
2
Ca/ℽ/Al O 3 Corn oil Methanol 12:1 65 5 87.89 – 34.64 118
2
ℽ‐Al O /KI Palm oil Methanol 14:1 60 4 98 – 79 119
3
2
MgO/MgAl O (untreated and Sunflower oil Methanol 12:1 110 3 79.30 – 91.10 120
2
3
treated with plasma)
MgO/MgAl O (untreated and Sunflower oil Methanol 12:1 110 3 95.70 – 96.50 121
2
3
treated with plasma)
Cr/Ca/ℽ‐Al O 3 Cooking oil Methanol 18:1 65 3 92.79 – 78.29 122
2
Mg/MgFe O 4 Sunflower oil Methanol 12:1 110 4 91.20 – 82.40 123
2
KOH/Fe O @Al O 3 Canola oil Methanol 12:1 110 4 98.80 – 88.40 124
4
2
3
CaO/CuFe O 4 Chicken fat Methanol 15:1 70 4 94.52 125
2
25%MoO /B‐ZSM‐5 Oleic acid Methanol 20:1 160 6 98 – 93 126
3
reuse of catalysts, contributing to the sustainability of low surface area limits the availability of active sites,
the biodiesel production process. reducing catalytic efficiency, particularly in reactions
One of the most popular methods for describing demanding high activity. Mass transfer limitations can
nanocatalysts is X-ray diffraction, which is used to also hinder performance, especially when using large
ascertain the catalyst’s composition and crystallinity. catalyst particles or operating at low fluid velocities, as
Analyzing the shape of nanocatalysts is made easier they restrict reactant access to active sites and impede
with the use of scanning electron microscopy. Thermal product removal, slowing overall reaction kinetics. In
stability and breakdown characteristics of catalyst addition, certain reaction components, such as water or
samples are assessed using thermogravimetric analysis, acidic compounds, can induce undesirable side reactions
while surface functional groups are identified using (e.g., soap formation in esterification), compromising
Fourier transform infrared spectroscopy. 51 process efficiency.
One of the benefits of heterogeneous nanocatalysts
is their ease of separation from the reaction mixture, as 3. Biomass waste-based catalysts and their
they exist in a different phase compared to the reactants function in biodiesel production
and products. This simplifies product purification and
enables continuous processing, while also allowing for Biomass wastes that include elements, such as
catalyst reuse with minimal activity loss, significantly carbon, silicon, calcium, magnesium, potassium, and
lowering operational costs. In addition, heterogeneous phosphorus have been investigated for the development
nanocatalysts can deliver high conversion efficiencies of inexpensive and ecologically friendly catalysts
in many reactions, often enabling processes to proceed (Figure 2). This section explores the production of
under milder temperature and pressure conditions solid catalysts from a variety of biomass waste sources,
compared to homogeneous systems. This not only including ashes, discarded shells, bones, and carbon-
reduces energy requirements but also enhances process based materials from agricultural leftovers, and assesses
safety. Furthermore, many heterogeneous catalysts are how well they work in the transesterification process.
cost-effective to manufacture, and their robust nature The best catalysts for transesterification processes
permits extended reuse without substantial degradation. are highlighted by comparing the catalytic activity of
These attributes, durability, reusability, and operational catalysts made from various biomass types.
efficiency, make them particularly advantageous for
large-scale industrial applications. 3.1. Shell wastes
However, these catalysts also have several When transesterifying low FFA feedstocks, CaO is
disadvantages. The performance of a heterogeneous a very efficient basic heterogeneous catalyst that is
catalyst is highly dependent on its surface area, as a frequently utilized because of its low solubility, low
Volume 22 Issue 5 (2025) 8 doi: 10.36922/AJWEP025130095