Page 91 - BH-2-4
P. 91
Brain & Heart Human DPSCs attenuated amyotrophic lateral sclerosis in mice
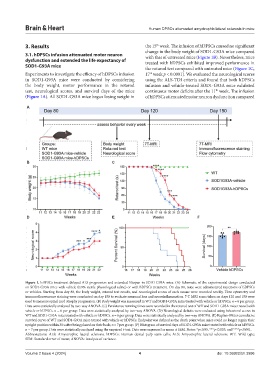
3. Results the 15 week. The infusion of hDPSCs caused no significant
th
change in the body weight of SOD1-G93A mice compared
3.1. hDPSCs infusion attenuated motor neuron with that of untreated mice (Figure 1B). Nevertheless, mice
dysfunction and extended the life expectancy of treated with hDPSCs exhibited improved performance in
SOD1-G93A mice the rotarod test compared with untreated mice (Figure 1C,
Experiments to investigate the efficacy of hDPSCs infusion 17 week p < 0.0001). We evaluated the neurological scores
th
in SOD1-G93A mice were conducted by considering using the ALS-TDI criteria and found that both hDPSCs
the body weight, motor performance in the rotarod infusion and vehicle-treated SOD1-G93A mice exhibited
test, neurological scores, and survival days of the mice continuous motor deficits after the 11 week. The infusion
th
(Figure 1A). All SOD1-G93A mice began losing weight in of hDPSCs attenuated motor neuron dysfunction compared
A
B C
D E F
Figure 1. hDPSCs treatment delayed ALS progression and extended lifespan in SOD1-G93A mice. (A) Schematic of the experimental design conducted
on SOD1-G93A mice with vehicle (0.9% sterile physiological saline) or with hDPSCs treatment. On day 80, mice were administered injections of hDPSCs
or vehicles. Starting from day 80, the body weight, rotarod test results, and neurological scores of each mouse were recorded weekly. Flow cytometry and
immunofluorescence staining were conducted on day 150 to evaluate neuronal loss and neuroinflammation. 7-T MRI scans taken on days 120 and 150 were
used to measure spinal cord atrophy progression. (B) Body weight was measured in WT and SOD1-G93A mice treated with vehicle or hDPSCs. n = 6 per group.
Data were statistically analyzed by two-way ANOVA. (C) Persistence running times were recorded in the rotarod test of WT and SOD1-G93A mice treated with
vehicle or hDPSCs. n = 6 per group. Data were statistically analyzed by two-way ANOVA. (D) Neurological deficits were evaluated using behavioral scores in
WT and SOD1-G93A mice treated with vehicle or hDPSCs. n = 6 per group. Data were statistically analyzed by two-way ANOVA. (E) Kaplan–Meier cumulative
survival curve of WT and SOD1-G93A mice treated with vehicle or hDPSCs. Endpoint was defined as the death point when mice could no longer regain their
upright position within 30 s after being placed on their back; n = 7 per group. (F) Histogram of survival days of SOD1-G93A mice treated with vehicle or hDPSCs.
n = 7 per group. Data were statistically analyzed using the unpaired t-test. Data were expressed as mean ± SEM. Notes: *p<0.05, ***p<0.005, and ****p<0.001.
Abbreviations: ALS: Amyotrophic lateral sclerosis; hDPSCs: Human dental pulp stem cells; ALS: Amyotrophic lateral sclerosis; WT: Wild type;
SEM: Standard error of mean; ANOVA: Analysis of variance.
Volume 2 Issue 4 (2024) 4 doi: 10.36922/bh.3996