Page 13 - BH-3-1
P. 13
Brain & Heart TAVR in low gradient aortic stenosis
A States, approved TAVR valves include SAPIEN 3 and
SAPIEN 3 Ultra from Edwards Lifesciences, Evolut Pro+
from Medtronic, and Lotus Valve System from Boston
Scientific, which was later recalled owing to issues in the
delivery system. The SAPIEN 3 valve, which was tested
in the PARTNER 3 trial, outperformed SAVR in low-risk
patients and received Food and Drug Administration
approval in June 2017. Compared with SAVR, the SAPIEN
3 valve, a balloon-expandable device, has shown superior
outcomes in low-risk patients in terms of mortality,
stroke, rehospitalization, atrial fibrillation, and severe
bleeding. However, it is associated with higher incidences
31
of new left bundle branch block (LBBB) and paravalvular
regurgitation. The SAPIEN X4, which is the latest
31
iteration of the Edwards THV, boasts some advancements
such as enhanced anticalcification technology, adjustable
B
sizing, and improved delivery. The ongoing ALLIANCE
study will further assess its safety and efficacy.
The Evolut FX SE, which is Medtronic’s most recent
Evolut THV, features enhancements for more predictable
implant depth and improved commissural alignment. It
retains key features from its predecessor, the Evolut Pro+,
including a nitinol delivery catheter capsule for recapturing
and repositioning, optimized radial force, and an inline
sheath for diverse vascular anatomy. Initial experiences
with the Evolut FX system demonstrated enhanced implant
depth and commissural alignment compared with previous
models. Although there is currently no evidence comparing
balloon-expandable and self-expanding valves in patients
with LFLGAS, some data regarding the comparison of
these valves in the general population are available. Studies
C
comparing balloon-expandable and self-expanding
valves typically involve patients with older-generation
valves. These comparisons indicate that self-expanding
valves outperform balloon-expandable valves in terms of
pacemaker rates and paravalvular regurgitation for small
annuli. However, mortality outcomes remain unclear, and
these findings may not be applicable to newer valve designs
aimed at mitigating such complications. 32
6. Complications and challenges
As with any procedure, it is crucial to be cognizant of and
mitigate the risks associated with TAVR. Although much
of the procedural risk has reduced with the optimization
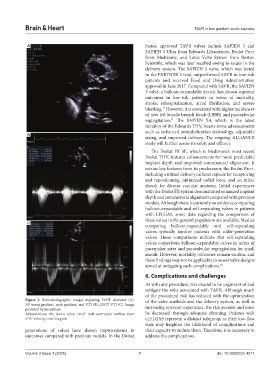
Figure 3. Echocardiographic images depicting LVOT diameter (A), of the valve scaffolds and the delivery system, as well as
AV mean gradient, peak gradient, and VTI (B), LVOT VTI (C). Image increasing operator experience, the risk persists and must
provided by the authors
Abbreviations: AV: Aortic valve; LVOT: Left ventricular outflow tract; be decreased through adequate planning. Patients with
VTI: Velocity time integrals. cLFLGAS represent a distinct subgroup, as their low-flow
state may heighten the likelihood of complications and
generations of valves have shown improvements in their capacity to endure them. Therefore, it is necessary to
outcomes compared with previous models. In the United address the complications.
Volume 3 Issue 1 (2025) 7 doi: 10.36922/bh.4017