Page 90 - BH-3-1
P. 90
Brain & Heart Brain lesions with PROKR2 microduplication
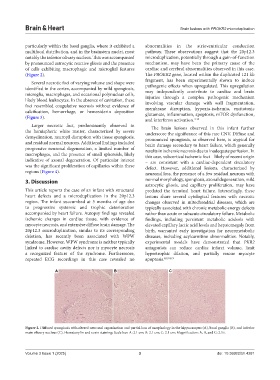
particularly within the basal ganglia, where it exhibited a abnormalities in the atrioventricular conduction
multifocal distribution, and in the brainstem nuclei, most pathway. These observations suggest that the 20p12.3
notably the inferior olivary nucleus. This was accompanied microduplication, potentially through a gain-of-function
by pronounced astrocytic reactive gliosis and the presence mechanism, may have been the primary cause of the
of cells exhibiting macrophagic and microglial features cardiac and cerebral abnormalities observed in this case.
(Figure 2). The PROKR2 gene, located within the duplicated 121 kb
Several necrotic foci of varying volume and shape were fragment, has been experimentally shown to induce
identified in the cortex, accompanied by mild spongiosis, pathogenic effects when upregulated. This upregulation
microglia, macrophages, and occasional polynuclear cells, may independently contribute to cardiac and brain
injuries through a complex pathogenic mechanism
likely blood leukocytes. In the absence of cavitation, these involving vascular damage with wall fragmentation,
foci resembled coagulative necrosis without evidence of membrane disruption, hypoxia-ischemia, excitotoxic
calcification, hemorrhage, or hemosiderin deposition glutamate, inflammation, apoptosis, mTOR dysfunction,
(Figure 3). and interferon activation. 3-15
Larger necrotic foci, predominantly observed in The brain lesions observed in this infant further
the hemispheric white matter, characterized by severe underscore the significance of this rare CNV. Diffuse and
demyelination, neuropil disruption with tissue spongiosis, pronounced spongiosis, as observed here, is atypical for
and residual normal neurons. Additional findings included brain damage secondary to heart failure, which generally
progressive neuronal degeneration, a limited number of results in ischemic necrosis due to inadequate perfusion. In
macrophages, and the presence of small spheroids, likely this case, subcortical ischemic foci – likely of recent origin
indicative of axonal degeneration. Of particular interest – are consistent with a cardiac-dependent circulatory
was the significant proliferation of capillaries within these defect. However, additional lesions, characterized by
regions (Figure 4). neuronal loss, the presence of a few residual neurons with
3. Discussion normal morphology, spongiosis, axonal degeneration, mild
astrocytic gliosis, and capillary proliferation, may have
This article reports the case of an infant with structural predated the terminal heart failure. Interestingly, these
heart defects and a microduplication in the 20p12.3 lesions share several cytological features with necrotic
region. The infant succumbed at 5 months of age due changes observed in mitochondrial diseases, which are
to progressive systemic and trophic deterioration typically associated with chronic metabolic energy defects
accompanied by heart failure. Autopsy findings revealed rather than acute or subacute circulatory failure. Metabolic
ischemic changes in cardiac tissue, with evidence of findings, including persistent metabolic acidosis with
myocyte necrosis, and extensive diffuse brain damage. The elevated capillary lactic acid levels and hepatomegaly from
20p12.3 microduplication, similar to its corresponding birth, warranted early investigation for neurometabolic
deletion, has recently been associated with WPW diseases, including acylcarnitine abnormalities. Notably,
syndrome. However, WPW syndrome is neither typically experimental models have demonstrated that PKR2
linked to cardiac cavity defects nor is myocyte necrosis antagonists can reduce cardiac infarct volume, limit
a recognized feature of the syndrome. Furthermore, hypertrophic dilation, and partially rescue myocyte
repeated ECG recordings in this case revealed no apoptosis. 8,9,18,19
A B C
Figure 2. Diffused spongiosis with altered neuronal organization and partial loss of morphology in the hippocampus (A), basal ganglia (B), and inferior
main olivary nucleus (C). Hematoxylin and eosin staining; Scale bar: A: 2.1 cm; B: 2.1 cm; C: 2.1 cm; Magnification: A, B, and C: 2.5×.
Volume 3 Issue 1 (2025) 3 doi: 10.36922/bh.4281