Page 28 - GTM-3-4
P. 28
Global Translational Medicine Metabolic dysfunction in vascular senescence
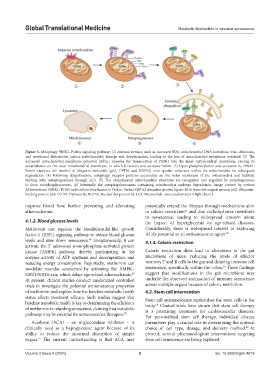
Figure 3. Mitophagy PINK1–Parkin signaling pathway; (1) external stresses, such as increased ROS, mitochondrial DNA mutations, toxic chemicals,
and nutritional deficiencies, induce mitochondrial damage and depolarization, leading to the loss of mitochondrial membrane potential. (2) The
decreased mitochondrial membrane potential (ΔΨm) impedes the translocation of PINK1 into the inner mitochondrial membrane, causing its
accumulation on the outer mitochondrial membrane, in which it recruits and activates Parkin. (3) Upon phosphorylation and activation by PINK1,
Parkin catalyzes the transfer of ubiquitin molecules (p62, OPTN, and NDP52) onto specific substrates within the mitochondria for subsequent
degradation. (4) Following ubiquitination, autophagy receptor proteins accumulate on the outer membrane of the mitochondria and facilitate
binding with autophagosomes through LC3. (5) The ubiquitinated mitochondrial substrates are recognized and engulfed by autophagosomes
to form autophagolysosomes. (6) Eventually the autophagolysosomes containing mitochondria undergo degradation. Image created by authors
Abbreviations: PINK1: PTEN-induced putative kinase 1; Parkin: Parkin RBR E3 ubiquitin-protein ligase; ROS: Reactive oxygen species; p62: Ubiquitin-
binding protein p62; OPTN: Optineurin; NDP52: Nuclear dot protein 52; LC3: Microtubule-associated protein 1 light chain 3.
improve blood flow, further preventing and alleviating potentially extend the lifespan through mechanisms akin
atherosclerosis. to caloric restriction and that carbohydrates contribute
61
to senescence, leading to widespread concern about
4.1.2. Blood glucose levels the impact of hyperglycemia on age-related diseases.
Metformin can regulate the insulin/insulin-like growth Considerably, there is widespread interest in exploring
factor-1 (IGF1) signaling pathway to reduce blood glucose ACA’s potential as an antisenescence agent. 62
levels and slow down senescence. Simultaneously, it can 4.1.3. Caloric restriction
58
activate the 5' adenosine monophosphate-activated protein
kinase (AMPK) pathway, thereby participating in the Caloric restriction diets lead to alterations in the gut
enzyme activity of ATP synthesis and decomposition and microbiota of mice, reducing the levels of effector
reducing energy consumption. Reportedly, metformin can memory T and B cells in the gut and delaying immune cell
63
modulate vascular senescence by activating the AMPK– senescence, specifically within the colon. These findings
SIRT1/SIRT6 axis, which delays age-related atherosclerosis. suggest that modifications in the gut microbiota may
59
At present, clinical studies conduct randomized controlled underlie the observed attenuation of immune senescence
trials to investigate the potential antisenescence properties across multiple organs because of caloric restriction.
of metformin and explore how the baseline metabolic health 4.2. Stem cell intervention
status affects treatment efficacy. Such studies suggest that Stem cell antisenescence replenishes the stem cells in the
baseline metabolic health is key to determining the efficiency body. Clinical trials have shown that stem cell therapy
64
of metformin in retarding senescence, claiming that metabolic is a promising treatment for cardiovascular diseases.
pathways may be essential for antisenescence therapies. 60
For personalized stem cell therapy, individual disease
Acarbose (ACA) – an α-glucosidase inhibitor – is parameters play a crucial role in determining the optimal
clinically used as a hypoglycemic agent because of its choice of cell type, dosage, and delivery method. At
65
ability to reduce the intestinal absorption of simple present, several pharmacological interventions targeting
sugars. The current understanding is that ACA may stem cell senescence are being explored.
61
Volume 3 Issue 4 (2024) 8 doi: 10.36922/gtm.4619