Page 26 - GTM-3-4
P. 26
Global Translational Medicine Metabolic dysfunction in vascular senescence
and adenosine triphosphate (ATP) synthase, thereby senescence. Sirt6 has also been associated with the
41
inhibiting mitochondrial biogenesis and causing energy improvement of dyslipidemia, cellular senescence, and left
metabolism disorders (Figure 2). Furthermore, reduced ventricular hypertrophy. 42
31
SIRT1 activity can inhibit mitochondrial autophagy
(mitophagy), accumulating damaged mitochondria and 3.2. Mitochondrial autophagy disorder
decreasing antioxidant enzymes, such as SOD2, which Mitochondria are essential organelles for ATP production
results in increased oxidative stress, vascular stiffness, and play a critical role in maintaining cellular energy
vascular senescence, and a higher risk of atherosclerosis. homeostasis. Mitochondrial dysfunction can expedite
38
43
Moreover, knocking out SIRT1 in MSCs in mice leads to fat senescence by disrupting the cytoplasmic NAD /reduced
+
tissue loss, indicating that SIRT1 coordinates antioxidant nicotinamide adenine dinucleotide (NADH) ratio and
responses and inhibits cellular senescence to protect increasing ROS production ROS. Impaired mitophagy,
adipogenesis. SIRT1 mitigates cellular senescence by which is crucial for removing damaged mitochondria,
39
deacetylating cell cycle-related proteins, such as p53. accumulates dysfunctional mitochondria, which further
Furthermore, it inhibits inflammatory pathways, such as exacerbates oxidative stress and accelerates the senescence
NF-κB, for reducing the release of SASP factors alleviating process. 44
inflammation, and delays cellular senescence. Research
39
has also been conducted on other members of the SIRT Mitophagy can be broadly categorized into ubiquitin-
family. For example, Sirt2 – as an epigenetic regulator – dependent and non-ubiquitin-dependent pathways.
can regulate vascular senescence through the cytoplasm– The ubiquitin-dependent pathway, which is the most
mitochondrial shuttle mechanism. 40 Sirt3 maintains extensively studied, primarily relies on the ubiquitination
metabolic homeostasis and the redox balance through of mitochondrial surface proteins to facilitate mitophagy. 45
deacetylation, providing a new perspective for treating The key proteins in the ubiquitin-dependent pathway are
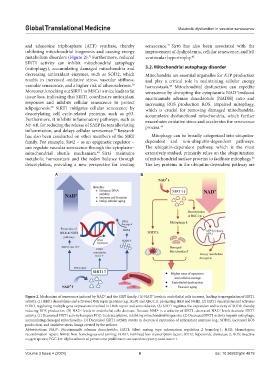
A B
Figure 2. Mechanism of senescence induced by NAD and the SIRT family; (A) NAD levels in endothelial cells increase, leading to upregulation of SIRT1
+
+
activity. (1) SIRT1 deacetylates and activates DNA repair proteins (e.g., Ku70 and XRCC1), promoting HRR and NHEJ. (2) SIRT1 deacetylates and activates
FOXO, regulating multiple gene expressions involved in DNA repair and antioxidation. (3) SIRT1 regulates the expression and activity of SOD2, thereby
reducing ROS production. (B) NAD levels in endothelial cells decrease. Because NAD is a cofactor of SIRT1, decreased NAD levels decrease SIRT1
+
+
+
activity. (1) Decreased SIRT1 activity hampers PGC-1α deacetylation, inhibiting mitochondrial biogenesis. (2) Decreased SIRT1 activity impairs mitophagy,
accumulating damaged mitochondria. (3) Decreased SIRT1 activity results in decreased expression of antioxidant enzymes (e.g., SOD2), increased ROS
production, and oxidative stress. Image created by the authors.
Abbreviations: NAD : Nicotinamide adenine dinucleotide; SIRT1: Silent mating–type information regulation 2 homolog-1; HRR: Homologous
+
recombination repair; NHEJ: Non-homologous end joining; FOXO: Forkhead box transcription factor; SOD2: Superoxide dismutase 2; ROS: Reactive
oxygen species; PGC-1α: Alpha subunit of peroxisome proliferator–activated receptor-γ coactivator-1.
Volume 3 Issue 4 (2024) 6 doi: 10.36922/gtm.4619