Page 23 - GTM-3-4
P. 23
Global Translational Medicine Metabolic dysfunction in vascular senescence
p53 (p53)/p21 and cyclin-dependent kinase inhibitor 2A vasoconstriction, maintenance of blood vessel wall integrity,
(p16 INK4a )/retinoblastoma pathways. These pathways act as and blood pressure regulation. Endothelial cell senescence
critical checkpoints that prevent the cells from continuing remarkably contributes to vascular senescence by inducing
to divide at specific thresholds related to time-dependent or heightened secretory and proinflammatory cytokine activities,
intrinsic signals. Thus, senescence is a response to external as well as exhibiting an enlarged and flattened appearance,
7
damage and an intrinsic physiological mechanism evolving which is a characteristic of cellular senescence. Consequently,
because of the accumulation of cell cycle arrest signals over the presence of senescent endothelial cells impairs endothelial
time or senescence is not exclusively a response to external function and reduces vasodilation ability, resulting in ischemia
damage; it is an intrinsic physiological mechanism that across multiple organs. In addition, these cells display increased
evolves because of the accumulation of cell cycle arrest polyploidy levels, along with elevated senescence-associated
signals over time. Typical features of senescent cells include β-galactosidase activity, and telomere shortening. Moreover,
9
cell cycle arrest and the development of a senescence- the SASP in endothelial cells underscores age-related
associated secretory phenotype (SASP), characterized by the alterations that play a pivotal role in arterial dysfunction. 10
secretion of proinflammatory chemokines (e.g., chemokine
[C–X–C motif] ligand [CXCL]1 and CXCL8), cytokines Endothelial cell senescence is an important predisposing
11
(e.g., interleukin [IL]-6, IL-1α, IL-8, and tumor necrosis factor for various cardiovascular diseases. For example,
factor-alpha [TNF-α]), growth factors, and reactive oxygen endothelial cell senescence can locally recruit macrophages
species (ROS). Cellular senescence underlies and triggers to the subendothelium, in which they transform into
8
the development of various diseases, particularly age-related foam cells owing to SASP alterations. In addition,
ones, and cellular senescence is reportedly involved in cardiac senescent endothelial cells can modulate the phenotypic
remodeling, atherosclerosis, and heart failure. Next, we transformation of smooth muscle and inflammatory cells
5
will focus on the mechanisms underlying endothelial cells, through exosome secretions, thereby promoting plaque
12
smooth muscle cells, macrophages, and stem cell senescence formation and increasing atherosclerosis susceptibility.
(Figure 1). Senescent endothelial cells also upregulate pathways
associated with arterial remodeling (e.g., transforming
2.2.1. Endothelial cell senescence growth factor β and matrix metalloproteinase [MMP]
Vascular endothelial cells are located in the intima of pathways), which may promote atherosclerosis. As
blood vessels, exerting regulatory effects over vasodilation, senescent markers, p53 and p21 usually increase in
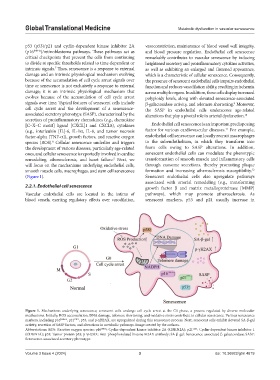
Figure 1. Mechanisms underlying senescence; senescent cells undergo cell cycle arrest at the G0 phase, a process regulated by diverse molecular
mechanisms. Initially, ROS accumulation, DNA damage, telomere shortening, and oxidative stress contribute to cellular senescence. Various senescence
markers, including p16 INK4A , p21 CIP1 , p53, and p-γH2AX, are upregulated during this senescence process. Next, senescent cells exhibit elevated SA-β-gal
activity, secretion of SASP factors, and alterations in metabolic pathways. Image created by the authors.
Abbreviations: ROS: Reactive oxygen species; p16 INKA : Cyclin-dependent kinase inhibitor 2A (CDKN2A); p21 CIP1 : Cyclin-dependent kinase inhibitor 1
(CDKN1A); p53: Tumor protein p53; p-γH2AX: Anti-phosphorylated histone H2AX antibody; SA-β-gal: Senescence-associated β-galactosidase; SASP:
Senescence-associated secretory phenotype.
Volume 3 Issue 4 (2024) 3 doi: 10.36922/gtm.4619