Page 41 - GTM-4-2
P. 41
Global Translational Medicine SPION for cancer theranostics
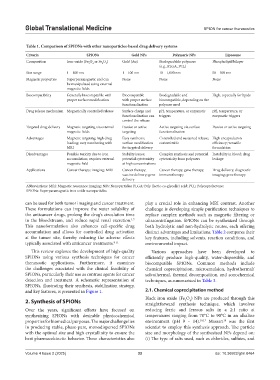
Table 1. Comparison of SPIONs with other nanoparticles‑based drug delivery systems
Criteria SPIONs Gold NPs Polymeric NPs Liposome
Composition Iron-oxide (Fe O or Fe O ) Gold (Au) Biodegradable polymers Phospholipid bilayer
3 4 2 3
(e.g., PLGA, PCL)
Size range 1 – 100 nm 1 – 100 nm 10 – 1,000 nm 50 – 500 nm
Magnetic properties Superparamagnetic and can None None None
be manipulated using external
magnetic fields
Biocompatibility Generally biocompatible with Biocompatible Biodegradable and High, especially for lipids
proper surface modification with proper surface biocompatible, depending on the
functionalization polymer used
Drug release mechanism Magnetically controlled release Surface charge and pH, temperature, or enzymatic pH, temperature, or
functionalization can triggers enzymatic triggers
control the release
Targeted drug delivery Magnetic targeting via external Passive or active Active targeting via surface Passive or active targeting
magnetic fields targeting functionalization
Advantages Magnetic targeting; high drug Easy synthesis; Controlled and sustained release; High encapsulation
loading; easy monitoring with surface modification customizable efficiency; versatile
MRI for targeted delivery formulation
Disadvantages Possible toxicity due to iron Stability issues; Complex synthesis and potential Instability in blood; drug
accumulation; requires external potential cytotoxicity cytotoxicity from polymers leakage
magnetic field at high concentrations
Applications Cancer therapy; imaging; MRI Cancer therapy; Cancer therapy; gene therapy; Drug delivery; diagnostic
vaccine delivery; gene immunotherapy imaging; gene therapy
delivery
Abbreviations: MRI: Magnetic resonance imaging; NPs: Nanoparticles; PLGA: Poly (lactic-co-glycolic) acid; PCL: Polycaprolactone;
SPIONs: Superparamagnetic iron oxide nanoparticles.
can be used for both tumor imaging and cancer treatment. play a crucial role in enhancing MRI contrast. Another
These formulations can improve the water solubility of challenge is developing simple purification techniques to
the anticancer drugs, prolong the drug’s circulation time replace complex methods such as magnetic filtering or
in the bloodstream, and reduce rapid renal excretion. ultracentrifugation. SPIONs can be synthesized through
1,7
This nanoformulation also enhances cell-specific drug both hydrolytic and non-hydrolytic routes, each offering
accumulation and allows for controlled drug activation distinct advantages and limitations. Table 2 compares their
at the tumor site, thereby reducing the adverse effects key features, including solvents, reaction conditions, and
typically associated with anticancer treatments. 8-11 environmental impact.
This review explores the development of high-quality Various approaches have been developed to
SPIONs using various synthesis techniques for cancer efficiently produce high-quality, water-dispersible, and
theranostic applications. Furthermore, it examines biocompatible SPIONs. Common methods include
the challenges associated with the clinical feasibility of chemical coprecipitation, microemulsion, hydrothermal/
SPIONs, particularly their use as contrast agents for cancer solvothermal, thermal decomposition, and sonochemical
detection and treatment. A schematic representation of techniques, as summarized in Table 3.
SPIONs, illustrating their synthesis, stabilization strategy,
and key features, is presented in Figure 1. 2.1. Chemical coprecipitation method
Black iron oxide (Fe O ) NPs are produced through this
2. Synthesis of SPIONs straightforward synthesis technique, which involves
3
4
Over the years, significant efforts have focused on reducing ferric and ferrous salts in a 2:1 ratio at
synthesizing SPIONs with desirable physicochemical temperatures ranging from 70°C to 90°C in an alkaline
properties for biomedical purposes. The major challenge lies environment (pH 9 – 14). 16,17 Massart was the first
18
in producing stable, phase-pure, monodispersed SPIONs scientist to employ this synthesis approach. The particle
with the optimal size and high crystallinity to ensure the size and morphology of the synthesized NPs depend on:
best pharmacokinetic behavior. These characteristics also (i) The type of salts used, such as chlorides, sulfates, and
Volume 4 Issue 2 (2025) 33 doi: 10.36922/gtm.8464