Page 91 - IJB-10-1
P. 91
International Journal of Bioprinting 3D bioprinting for musculoskeletal system
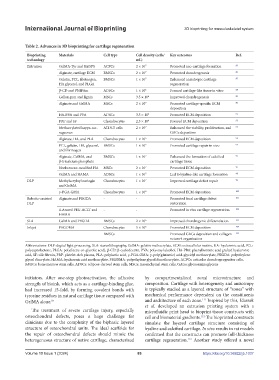
Table 2. Advances in 3D bioprinting for cartilage regeneration
Bioprinting Materials Cell type Cell density (cells/ Key outcomes Ref.
technology mL)
Extrusion GelMA-Tyr and Ru/SPS ACPCs 2 × 10 7 Promoted neo-cartilage formation 85
Alginate, cartilage ECM BMSCs 2 × 10 7 Promoted chondrogenesis 86
Gelatin, PCL, fibrinogen, BMSCs 1 × 10 7 Enhanced anisotropic cartilage 87
HA glycerol, and PLGA regeneration
β-CD and PNIPAm ADSCs 1 × 10 6 Formed cartilage-like tissue in vitro 88
Gellan gum and lignin MSCs 3.5 × 10 6 Improved chondrogenesis 89
Alginate and GelMA MSCs 2 × 10 7 Promoted cartilage-specific ECM 90
deposition
HA-PBA and PVA ADSCs 3.5 × 10 6 Promoted ECM deposition 91
PRP and SF Chondrocytes 2.5 × 10 6 Favored ECM deposition 92
Methacrylated kappa-car- ATDC5 cells 2 × 10 7 Enhanced the viability, proliferation, and 93
rageenan GAGs deposition
Alginate, HA, and PLA Chondrocytes 1 × 10 6 Promoted ECM deposition 94
PCL, gelatin, HA, glycerol, BMSCs 1 × 10 7 Promoted cartilage repair in vivo 95
and fibrinogen
Alginate, GelMA, and BMSCs 1 × 10 7 Enhanced the formation of calcified 96
β-tricalcium phosphate cartilage tissue
Norbornene-modified HA MSCs 2 × 10 7 Promoted ECM deposition 97
GelMA and HAMA ADSCs 1 × 10 7 Led to hyaline-like cartilage formation 98
DLP Methylacryloyl naringin Chondrocytes 1 × 10 7 Improved cartilage defect repair 99
and GelMA
γ-PGA-GMA Chondrocytes 1 × 10 6 Promoted ECM deposition 100
Robotic-assisted Alginate and PEGDA - - Promoted focal cartilage defect 101
DLP restoration
4-Armed PEG-ACLT and - - Promoted in vivo cartilage regeneration 102
HAMA
SLA GelMA and PEGDA BMSCs 2 × 10 6 Improved chondrogenic differentiation 103
Inkjet PEGDMA Chondrocytes 5 × 10 6 Promoted ECM deposition 104
- BMSCs - Promoted GAGs deposition and collagen 105
network organization
Abbreviations: DLP: digital light processing, SLA: stereolithography, GelMA: gelatin methacrylate, ECM: extracellular matrix, HA: hyaluronic acid, PCL:
polycaprolactone, PLGA: poly(lactic-co-glycolic acid), β-CD: β-cyclodextrin, PVA: polyvinyl alcohol, HA-PBA: phenylboronic acid grafted hyaluronic
acid, SF: silk fibroin, PRP: platelet-rich plasma, PLA: polylactic acid, γ-PGA-GMA: γ-poly(glutamic) acid-glycidyl methacrylate, PEGDA: polyethylene
glycol diacrylate, HAMA: hyaluronic acid methacrylate, PEGDMA: polyethylene glycol dimethacrylate, ACPCs: articular chondroprogenitor cells,
BMSCs: bone marrow stem cells, ADSCs: adipose-derived stem cells, MSCs: mesenchymal stem cells, GAGs: glycosaminoglycans
initiators. After one-step photoactivation, the adhesive by compartmentalized zonal microstructure and
strength of bioink, which acts as a cartilage-binding glue, composition. Cartilage with heterogeneity and anisotropy
had increased 15-fold, by forming covalent bonds with is typically studied as a layered structure of “zones” with
tyrosine residues in natural cartilage tissue compared with mechanical performance dependent on the constituents
111
GelMA alone. 85 and architecture of each zone. Inspired by this, Idaszek
et al. developed an extrusion printing system with a
The treatment of severe cartilage injury, especially microfluidic print head to bioprint tissue constructs with
osteochondral defects, poses a huge challenge for cell and biomaterial gradients. The bioprinted constructs
112
clinicians due to the complexity of the biphasic layered simulate the layered cartilage structure consisting of
structure of osteochondral units. The ideal scaffolds for hyaline and calcified cartilage. In vivo results in rat models
the repair of osteochondral defects should mimic the confirmed that the constructs can promote full-thickness
heterogeneous structure of native cartilage, characterized cartilage regeneration. Another study offered a novel
112
Volume 10 Issue 1 (2024) 83 https://doi.org/10.36922/ijb.1037