Page 94 - IJB-10-1
P. 94
International Journal of Bioprinting 3D bioprinting for musculoskeletal system
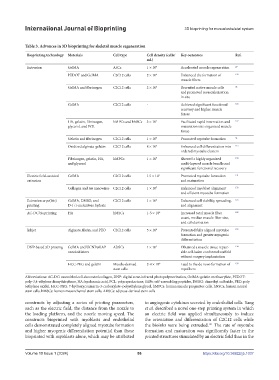
Table 3. Advances in 3D bioprinting for skeletal muscle regeneration
Bioprinting technology Materials Cell type Cell density (cells/ Key outcomes Ref.
mL)
Extrusion GelMA ASCs 1 × 10 7 Accelerated muscle regeneration 29
PEDOT and GelMA C2C12 cells 2 × 10 6 Enhanced the formation of 129
muscle fibers
GelMA and fibrinogen C2C12 cells 2 × 10 5 Recruited native muscle cells 36
and promoted revascularization
in situ
GelMA C2C12 cells - Achieved significant functional 126
recovery and higher muscle
forces
HA, gelatin, fibrinogen, hMPCs and hNSCs 3 × 10 7 Facilitated rapid innervation and 127
glycerol, and PCL maturation into organized muscle
tissue
Gelatin and fibrinogen C2C12 cells 1 × 10 7 Promoted myotube formation 31
Oxidized alginate-gelatin C2C12 cells 8 × 10 6 Enhanced cell differentiation into 115
ordered myotube clusters
Fibrinogen, gelatin, HA, hMPCs 1 × 10 7 Showed a highly organized 130
and glycerol multi-layered muscle bundle and
significant functional recovery
Electric field-assisted GelMA C2C12 cells 1.5 × 10 7 Promoted myotube formation 131
extrusion and maturation
Collagen and Au nanowires C2C12 cells 1 × 10 7 Enhanced myoblast alignment 132
and efficient myotube formation
Extrusion cryo(bio) GelMA, DMSO, and C2C12 cells 1 × 10 6 Enhanced cell viability, spreading, 125
printing D-(+)-melezitose hydrate and alignment
AC-DC bioprinting HA hMSCs 1-5 × 10 6 Increased total muscle fiber 128
count, median muscle fiber size,
and cellularization
Inkjet Alginate, fibrin, and PEO C2C12 cells 5 × 10 6 Presented fully aligned myotube 133
formation and greater myogenic
differentiation
DNP-based 3D printing GelMA and UCNP@LAP ADSCs 1 × 10 7 Obtained a muscle tissue repair- 134
nanoinitiators able cell-laden conformal scaffold
without surgery implantation
HCC-PEG and gelatin Muscle-derived 2-4 × 10 6 Lead to the de novo formation of 135
stem cells myofibers
Abbreviations: AC-DC: assembled cell-decorated collagen, DNP: digital near-infrared photopolymerization, GelMA: gelatin methacrylate, PEDOT:
poly-3,4-ethylene dioxythiophene, HA: hyaluronic acid, PCL: polycaprolactone, SAPs: self-assembling peptides, DMSO: dimethyl sulfoxide, PEO: poly
(ethylene oxide), HCC-PEG: 7-hydroxycoumarin-3-carboxylate–polyethylene glycol, hMPCs: human muscle progenitor cells, hNSCs: human neural
stem cells, hMSCs: human mesenchymal stem cells, ADSCs: adipose-derived stem cells
constructs by adjusting a series of printing parameters, to angiogenic cytokines secreted by endothelial cells. Yang
such as the electric field, the distance from the nozzle to et al. described a novel one-step printing system in which
the loading platform, and the nozzle moving speed. The an electric field was applied simultaneously to induce
constructs bioprinted with myoblasts and endothelial the orientation and differentiation of C2C12 cells while
cells demonstrated completely aligned myotube formation the bioinks were being extruded. The rate of myotube
131
and higher myogenic differentiation potential than those formation and maturation was significantly faster in the
bioprinted with myoblasts alone, which may be attributed printed structures stimulated by an electric field than in the
Volume 10 Issue 1 (2024) 86 https://doi.org/10.36922/ijb.1037