Page 97 - IJB-10-1
P. 97
International Journal of Bioprinting 3D bioprinting for musculoskeletal system
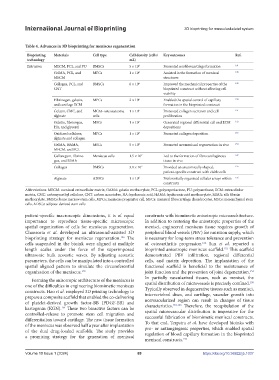
Table 4. Advances in 3D bioprinting for meniscus regeneration
Bioprinting Materials Cell type Cell density (cells/ Key outcomes Ref.
technology mL)
Extrusion MECM, PCL, and PU BMSCs 5 × 10 6 Promoted neofibrocartilage formation 141
GelMA, PCL, and MFCs 1 × 10 6 Assisted in the formation of meniscal 148
MECM structures
Collagen, PCL, and BMSCs 6 × 10 6 Improved the mechanical properties of the 149
CNT bioprinted construct without affecting cell
viability
Fibrinogen, gelatin, MPCs 2 × 10 6 Enabled the spatial control of capillary 150
and cartilage ECM formation in the bioprinted construct
Gelatin, CMC, and MG63-osteosarcoma 1 × 10 5 Promoted collagen secretion and cell 151
alginate cells proliferation
Gelatin, fibrinogen, MSCs 1 × 10 7 Generated regional differential cell and ECM 152
HA, and glycerol depositions
Oxidized cellulose, MFCs 1 × 10 7 Promoted collagen deposition 153
alginate and collagen
GelMA, HAMA, MSCs 5 × 10 5 Promoted neomeniscal regeneration in vivo 154
MECM, and PCL
Gellan gum, fibrino- Meniscus cells 1.5 × 10 7 Led to the formation of fibrocartilaginous 147
gen, and SilMA tissue in vivo
Collagen BMSCs 3.8 × 10 7 Provided an anatomically shaped, 155
patient-specific construct with viable cells
Alginate ADSCs 1 × 10 6 Preferentially organized cellular arrays within 156
constructs
Abbreviations: MECM: meniscal extracellular matrix, GelMA: gelatin methacrylate, PCL: polycaprolactone, PU: polyurethane, ECM: extracellular
matrix, CMC: carboxymethyl cellulose, CNT: carbon nanotubes, HA: hyaluronic acid, HAMA: hyaluronic acid methacrylate, SilMA: silk fibroin
methacrylate, BMSCs: bone marrow stem cells, MPCs: meniscus progenitor cell, MFCs: meniscal fibrocartilage chondrocytes, MSCs: mesenchymal stem
cells, ADSCs: adipose-derived stem cells
patient-specific macroscopic dimensions, it is of equal constructs with biomimetic anisotropic microarchitecture.
importance to reproduce tissue-specific microscopic In addition to restoring the anisotropic properties of the
spatial organization of cells for meniscus regeneration. menisci, engineered meniscus tissue requires growth of
Chansoria et al. developed an ultrasound-assisted 3D peripheral blood vessels (PBV) for nutrition supply, which
bioprinting strategy for meniscus regeneration. The is necessary for long-term stress tolerance and prevention
156
cells suspended in the bioink were aligned at multiple of osteoarthritis progression. Sun et al. reported a
162
length scales under the force of the superimposed bioprinted anisotropic meniscus scaffold. This scaffold
152
ultrasonic bulk acoustic waves. By adjusting acoustic demonstrated PBV infiltration, regional differential
parameters, the cells can be manipulated into a controlled cells, and matrix deposition. The implantation of the
spatial aligned pattern to simulate the circumferential functional scaffold is beneficial to the maintenance of
organization of the meniscus. joint function and the prevention of joint degeneration.
152
156
In partially vascularized tissues, such as menisci, the
Forming the anisotropic architecture of the meniscus is 150
one of the difficulties in engineering biomimetic meniscus spatial distribution of microvessels is precisely confined.
Typically observed in degenerative tissues such as menisci,
constructs. Hao et al. employed 3D printing technology to intervertebral discs, and cartilage, vascular growth into
prepare a composite scaffold that enabled the co-delivering nonvascularized region can result in changes of tissue
of platelet-derived growth factor-BB (PDGF-BB) and characteristics. 163-166 Therefore, the recapitulation of the
kartogenin (KGN). These two bioactive factors can be spatial microvascular distribution is imperative for the
154
controlled-release to promote stem cell migration and successful fabrication of biomimetic meniscal constructs.
differentiation toward cartilage. The new tissue formation To that end, Terpstra et al. have developed bioinks with
of the meniscus was observed half a year after implantation pro- or antiangiogenic properties, which enabled spatial
of the dual drug-loaded scaffolds. The study provides regulation of blood capillary formation in the bioprinted
a promising strategy for the generation of meniscal
meniscal constructs. 150
Volume 10 Issue 1 (2024) 89 https://doi.org/10.36922/ijb.1037