Page 64 - IJB-2-1
P. 64
Polyelectrolyte gelatin-chitosan hydrogel optimized for 3D bioprinting in skin tissue engineering
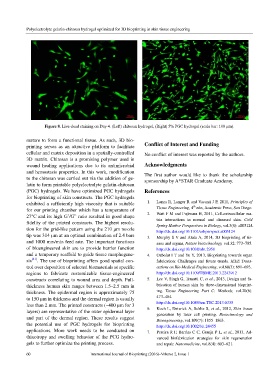
Figure 8. Live-dead staining on Day 4. (Left) chitosan hydrogel, (Right) 5% PGC hydrogel (scale bar: 100 μm).
mature to form a functional tissue. As such, 3D bio-
printing serves as an attractive platform to facilitate Conflict of Interest and Funding
cellular and matrix deposition in a spatially-controlled No conflict of interest was reported by the authors.
3D matrix. Chitosan is a promising polymer used in
wound healing applications due to its antimicrobial Acknowledgments
and hemostasis properties. In this work, modification The first author would like to thank the scholarship
to the chitosan was carried out via the addition of ge- sponsorship by A*STAR Graduate Academy.
latin to form printable polyelectrolyte gelatin-chitosan
(PGC) hydrogels. We have optimized PGC hydrogels References
for bioprinting of skin constructs. The PGC hydrogels
exhibited a sufficiently high viscosity that is suitable 1. Lanza R, Langer R and Vacanti J P, 2011, Principles of
th
for our printing chamber which has a temperature of Tissue Engineering, 4 edn, Academic Press, San Diego.
27°C and its high G’/G” ratio resulted in good shape 2. Watt F M and Fujiwara H, 2011, Cell-extracellular ma-
trix interactions in normal and diseased skin. Cold
fidelity of the printed constructs. The highest resolu- Spring Harbor Perspectives in Biology, vol.3(4): a005124.
tion for the grid-like pattern using the 210 μm nozzle http://dx.doi.org/10.1101/cshperspect.a005124
tip was 314 μm at an optimal combination of 2.4 bars 3. Murphy S V and Atala A, 2014, 3D bioprinting of tis-
and 1000 mm/min feed rate. The important functions sues and organs, Nature biotechnology, vol.32: 773–785.
of bioengineered skin are to provide barrier function http://dx.doi.org/10.1038/nbt.2958
and a temporary scaffold to guide tissue morphogene- 4. Ozbolat I T and Yu Y, 2013, Bioprinting towards organ
sis [41] . The use of bioprinting offers good spatial con- fabrication: Challenges and future trends. IEEE Trans-
trol over deposition of selected biomaterials at specific actions on Bio-Medical Engineering, vol.60(3): 691–693.
regions to fabricate customizable tissue-engineered http://dx.doi.org/10.1109/TBME.2013.2243912
constructs correlating to wound area and depth. Full- 5. Lee V, Singh G, Trasatti C, et al., 2013, Design and fa-
thickness human skin ranges between 1.5–2.5 mm in brication of human skin by three-dimensional bioprint-
thickness. The epidermal region is approximately 75 ing. Tissue Engineering Part C: Methods, vol.20(6):
to 150 µm in thickness and the dermal region is usually 473–484.
less than 2 mm. The printed constructs (~400 µm for 3 http://dx.doi.org/10.1089/ten.TEC.2013.0335
layers) are representative of the outer epidermal layer 6. Koch L, Deiwick A, Schlie S, et al., 2012, Skin tissue
generation by laser cell printing. Biotechnology and
and part of the dermal region. These results suggest Bioengineering, vol.109(7): 1855–1863.
the potential use of PGC hydrogels for bioprinting http://dx.doi.org/10.1002/bit.24455
applications. More work needs to be conducted on 7. Pereira R F, Barrias C C, Granja P L, et al., 2013, Ad-
thixotropy and swelling behavior of the PCG hydro- vanced biofabrication strategies for skin regeneration
gels to further optimize the printing process. and repair. Nanomedicine, vol.8(4): 603–621.
60 International Journal of Bioprinting (2016)–Volume 2, Issue 1