Page 60 - IJB-2-1
P. 60
Polyelectrolyte gelatin-chitosan hydrogel optimized for 3D bioprinting in skin tissue engineering
widths at varying printing pressures and feed rates
using ImageJ processing software. To demonstrate its
ability to fabricate a multi-layered hydrogel construct,
a 3-layered hydrogel construct with grid-like patterns
was fabricated by printing each layer of grid-like pat-
terns directly over the previous layer using an optimal
combination of feed rates and printing pressures.
2.6 Biocompatibility of PGC Hydrogels
To assess the biocompatibility of PGC hydrogels, PGC
hydrogels were manually casted followed by seeding
150,000 HFF-1 cells on surface of PGC hydrogels in
each of the 6-well plates (n = 5) and 2 mL of complete
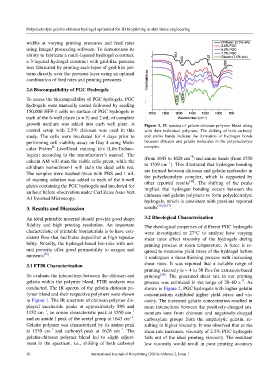
growth medium was added into each well plate. A Figure 1. IR spectra of gelatin-chitosan polymer blend along
control setup with 2.5% chitosan was used in this with their individual polymers. The shifting of both carbonyl
study. The cells were incubated for 4 days prior to and amino bands indicate the formation of hydrogen bonds
performing cell viability assay on Day 4 using Mole- between chitosan and gelatin molecules in the polyelectrolyte
®
cular Probes Live/Dead staining kits (Life-Techno- complex.
logies) according to the manufacturer’s manual. The –1
calcein AM will stain the viable cells green, while the (from 1643 to 1628 cm ) and amino bands (from 1550
–1
ethidium homodimer-1 will stain the dead cells red. to 1539 cm ). This illustrated that hydrogen bonding
The samples were washed twice with PBS and 1 mL are formed between chitosan and gelatin molecules in
of staining solution was added to each of the 6-well the polyelectrolyte complex, which is supported by
[36]
plates containing the PGC hydrogels and incubated for other reported results . The shifting of the peaks
implied that hydrogen bonding occurs between the
an hour before observation under Carl Zeiss Axio Vert. chitosan and gelatin polymers to form polyelectrolyte
A1 Inverted Microscopy.
hydrogels, which is consistent with previous reported
3. Results and Discussion results [30,36,37] .
An ideal printable material should provide good shape 3.2 Rheological Characterization
fidelity and high printing resolution. An important The rheological properties of different PGC hydrogels
characteristic of printable biomaterials is to have con- were investigated at 27°C to analyze how varying
sistent flow that facilitates deposition at high repeata- shear rates affect viscosity of the hydrogels during
bility. Notably, the hydrogel-based bio-inks with nat- printing process at room temperature. A force is re-
ural porosity offer good permeability to oxygen and quired to overcome yield stress of the hydrogel before
nutrients [35] . it undergoes a shear-thinning process with increasing
3.1 FTIR Characterization shear rates. It was reported that a suitable range of
printing viscosity is ~ 4 to 30 Pa⋅s for extrusion-based
To evaluate the interactions between the chitosan and printing [38] . The generated shear rate in our printing
–1
gelatin within the polymer blend, FTIR analysis was process was estimated in the range of 20–60 s . As
conducted. The IR spectra of the gelatin-chitosan po- shown in Figure 2, PGC hydrogels with higher gelatin
lymer blend and their respective polymers were shown concentrations exhibited higher yield stress and vis-
in Figure 1. The IR spectrum of chitosan polymer dis- cosity. The increased gelatin concentration resulted in
played saccharide peaks at approximately 896 and more interactions between the positively-charged am-
–1
–1
1152 cm , an amino characteristic peak at 1550 cm monium ions from chitosan and negatively-charged
–1
and an amide I peak of the acetyl group at 1643 cm . carboxylate groups from the ampholytic gelatin, re-
Gelatin polymer was characterized by its amino peak sulting in higher viscosity. It was observed that as the
–1
–1
at 1539 cm and carbonyl peak at 1628 cm . The shear rate increases, viscosity of 2.5% PGC hydrogels
gelatin-chitosan polymer blend led to slight adjust- falls out of the ideal printing viscosity. The resultant
ment in the spectrum, i.e., shifting of both carbonyl low viscosity would result in poor printing accuracy
56 International Journal of Bioprinting (2016)–Volume 2, Issue 1