Page 364 - IJB-10-2
P. 364
International Journal of Bioprinting 3D bioprinting for vascular regeneration
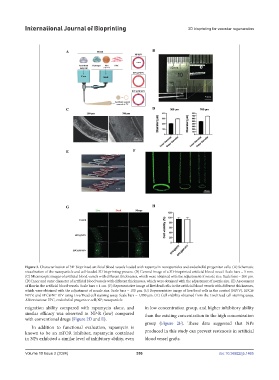
Figure 3. Characterization of 3D-bioprinted artificial blood vessels loaded with rapamycin-nanoparticles and endothelial progenitor cells. (A) Schematic
visualization of the nanoparticle and cell-loaded 3D bioprinting process. (B) General image of a 3D-bioprinted artificial blood vessel. Scale bars = 5 mm.
(C) Microscopic images of artificial blood vessels with different thicknesses, which were obtained with the adjustment of nozzle size. Scale bars = 200 μm.
(D) Inner and outer diameter of artificial blood vessels with different thicknesses, which were obtained with the adjustment of nozzle size. (E) Assessment
of flow in the artificial blood vessels. Scale bars = 1 cm. (F) Representative image of live/dead cells in the artificial blood vessels with different thicknesses,
which were obtained with the adjustment of nozzle size. Scale bars = 100 μm. (G) Representative image of live/dead cells in the control (NP/V), EPC@
NP/V, and EPC@NP-R/V using Live/Dead cell staining assay. Scale bars = 1,000 μm. (H) Cell viability obtained from the Live/Dead cell staining assay.
Abbreviations: EPC, endothelial progenitor cell; NP, nanoparticle.
migration ability compared with rapamycin alone, and in low concentration group, and higher inhibitory ability
similar efficacy was observed in NP-R (low) compared than the existing concentration in the high concentration
with conventional drugs (Figure 2D and E).
group (Figure 2F). These data suggested that NPs
In addition to functional evaluation, rapamycin is
known to be an mTOR inhibitor; rapamycin contained produced in this study can prevent restenosis in artificial
in NPs exhibited a similar level of inhibitory ability, even blood vessel grafts.
Volume 10 Issue 2 (2024) 356 doi: 10.36922/ijb.1465