Page 366 - IJB-10-2
P. 366
International Journal of Bioprinting 3D bioprinting for vascular regeneration
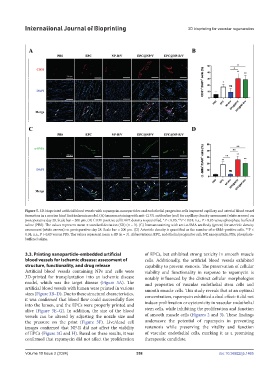
Figure 5. 3D-bioprinted artificial blood vessels with rapamycin-nanoparticles and endothelial progenitor cells improved capillary and arterial blood vessel
formation in a murine hind limb ischemia model. (A) Immunostaining with anti-CD31 antibodies (red) for capillary density assessment (white arrows) on
postoperative day 28. Scale bar = 200 µm. (B) CD31-positive cell/DAPI density is quantified. *P < 0.05; **P < 0.01; n.s., P > 0.05 versus phosphate-buffered
saline (PBS). The values represent mean ± standard deviation (SD) (n = 3). (C) Immunostaining with anti-α-SMA antibody (green) for arteriole density
assessment (white arrows) on postoperative day 28. Scale bar = 200 µm. (D) Arteriole density is quantified as the number of α-SMA-positive cells. **P <
0.01; n.s., P > 0.05 versus PBS. The values represent mean ± SD (n = 3). Abbreviations: EPC, endothelial progenitor cell; NP, nanoparticle; PBS, phosphate-
buffered saline.
3.3. Printing nanoparticle-embedded artificial of EPCs, but exhibited strong toxicity in smooth muscle
blood vessels for ischemic disease: assessment of cells. Additionally, the artificial blood vessels exhibited
structure, functionality, and drug release capability to prevent stenosis. The preservation of cellular
Artificial blood vessels containing NPs and cells were viability and functionality in response to rapamycin is
3D-printed for transplantation into an ischemic disease notably influenced by the distinct cellular morphologies
model, which was the target disease (Figure 3A). The and properties of vascular endothelial stem cells and
artificial blood vessels with lumen were printed in various smooth muscle cells. This study reveals that at an optimal
sizes (Figure 3B–D). Due to these structural characteristics, concentration, rapamycin exhibited a dual effect: it did not
it was confirmed that blood flow could successfully flow induce proliferation or cytotoxicity in vascular endothelial
into the lumen, and the EPCs were properly printed and
alive (Figure 3E–G). In addition, the size of the blood stem cells, while inhibiting the proliferation and function
vessels can be altered by adjusting the nozzle size and of smooth muscle cells (Figures 2 and 3). These findings
the pressure on the print (Figure 3F). Live/dead cell underscore the potential of rapamycin in preventing
images confirmed that NP-R did not affect the viability restenosis while preserving the vitality and function
of EPCs (Figure 3G and H). Based on these results, it was of vascular endothelial cells, marking it as a promising
confirmed that rapamycin did not affect the proliferation therapeutic candidate.
Volume 10 Issue 2 (2024) 358 doi: 10.36922/ijb.1465