Page 394 - IJB-10-2
P. 394
International Journal of Bioprinting Automated bioink mixer improves bioprinting
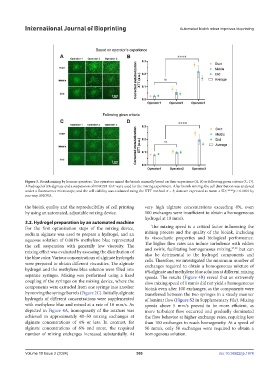
Figure 3. Bioink mixing by human operators. The operators mixed the bioink manually based on their experience (A, B) or following given criteria (C, D).
A hydrogel of 6% alginate and a suspension of HEK293-GFP were used for the mixing experiment. After bioink mixing, the cell distribution was analyzed
under a fluorescence microscope, and the cell viability was evaluated using the XTT method. n = 3; data are expressed as mean ± SD; ****p < 0.0001 by
one-way ANOVA.
the bioink quality and the reproducibility of cell printing very high alginate concentrations exceeding 8%, even
by using an automated, adjustable mixing device. 100 exchanges were insufficient to obtain a homogeneous
hydrogel at 10 mm/s.
3.2. Hydrogel preparation by an automated machine
For the first optimization steps of the mixing device, The mixing speed is a critical factor influencing the
sodium alginate was used to prepare a hydrogel, and an mixing process and the quality of the bioink, including
aqueous solution of 0.001% methylene blue represented its viscoelastic properties and biological performance.
the cell suspension with generally low viscosity. The The higher flow rates can induce turbulence with eddies
27,28
mixing effect was evaluated by assessing the distribution of and swirls, facilitating homogeneous mixing, but can
the blue color. Various concentrations of alginate hydrogels also be detrimental to the hydrogel components and
cells. Therefore, we investigated the minimum number of
were prepared to obtain different viscosities. The alginate exchanges required to obtain a homogeneous mixture of
hydrogel and the methylene blue solution were filled into 6% alginate and methylene blue solution at different mixing
separate syringes. Mixing was performed using a fixed speeds. The results (Figure 4B) reveal that an extremely
coupling of the syringes on the mixing device, where the slow mixing speed of 1 mm/s did not yield a homogeneous
components were extruded from one syringe into another bioink even after 100 exchanges, as the components were
by moving the syringe barrels (Figure 2C). Initially, alginate transferred between the two syringes in a steady manner
hydrogels of different concentrations were supplemented of laminar flow (Figure S2 in Supplementary File). Mixing
with methylene blue and mixed at a rate of 10 mm/s. As speeds above 5 mm/s proved to be more efficient, as
depicted in Figure 4A, homogeneity of the mixture was more turbulent flow occurred and gradually dominated
achieved in approximately 40–50 mixing exchanges at the flow behavior at higher exchange rates, requiring less
alginate concentrations of 4% or less. In contrast, for than 100 exchanges to reach homogeneity. At a speed of
alginate concentrations of 6% and more, the required 50 mm/s, only 50 exchanges were required to obtain a
number of mixing exchanges increased substantially. At homogenous solution.
Volume 10 Issue 2 (2024) 386 doi: 10.36922/ijb.1974