Page 416 - IJB-10-2
P. 416
International Journal of Bioprinting 3D-bioprinted macrophage inflammation model
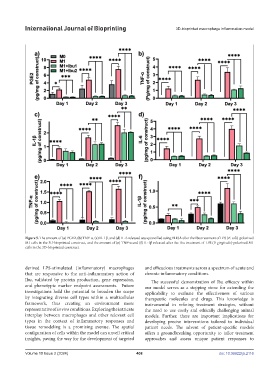
Figure 5. The amount of (a) PGE2, (b) TNF-α, (c) IL-1β, and (d) IL-6 released was quantified using ELISA after the Ibu treatment of LPS (E. coli)-polarized
M1 cells in the 3D-bioprinted construct, and the amount of (e) TNF-α and (f) IL-1β released after the Ibu treatment of LPS (P. gingivalis)-polarized M1
cells in the 3D-bioprinted construct.
derived LPS-stimulated (inflammatory) macrophages and efficacious treatments across a spectrum of acute and
that are responsive to the anti-inflammatory action of chronic inflammatory conditions.
Ibu, validated by protein production, gene expression, The successful demonstration of Ibu efficacy within
and phenotypic marker endpoint assessments. Future our model serves as a stepping stone for extending the
investigations hold the potential to broaden the scope applicability to evaluate the effectiveness of various
by integrating diverse cell types within a multicellular therapeutic molecules and drugs. This knowledge is
framework, thus creating an environment more instrumental in refining treatment strategies, without
representative of in vivo conditions. Exploring the intricate the need to use costly and ethically challenging animal
interplay between macrophages and other relevant cell models. Further, there are important implications for
types in the context of inflammatory responses and developing precise interventions tailored to individual
tissue remodeling is a promising avenue. The spatial patient needs. The advent of patient-specific models
configuration of cells within the model can unveil critical offers a groundbreaking opportunity to tailor treatment
insights, paving the way for the development of targeted approaches and assess unique patient responses to
Volume 10 Issue 2 (2024) 408 doi: 10.36922/ijb.2116