Page 493 - IJB-10-2
P. 493
International Journal of Bioprinting Bioprinted skin for testing of therapeutics
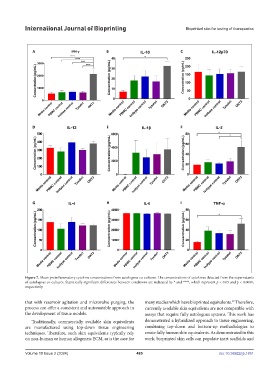
Figure 7. Mean proinflammatory cytokine concentrations from autologous co-cultures. The concentrations of cytokines detected from the supernatants
of autologous co-cultures. Statistically significant differences between conditions are indicated by * and ****, which represent p < 0.05 and p < 0.0001,
respectively.
that with reservoir agitation and microvalve purging, the many studies which have bioprinted equivalents. Therefore,
38
process can offer a consistent and automatable approach in currently available skin equivalents are not compatible with
the development of tissue models. assays that require fully autologous systems. This work has
Traditionally, commercially available skin equivalents demonstrated a hybridized approach to tissue engineering,
are manufactured using top-down tissue engineering combining top-down and bottom-up methodologies to
techniques. Therefore, such skin equivalents typically rely create fully human skin equivalents. As demonstrated in this
on non-human or human allogeneic ECM, as is the case for work, bioprinted skin cells can populate inert scaffolds and
Volume 10 Issue 2 (2024) 485 doi: 10.36922/ijb.1851