Page 489 - IJB-10-2
P. 489
International Journal of Bioprinting Bioprinted skin for testing of therapeutics
2.6. Assessment of adverse immune reactions of 1β), interleukin-2 (IL-2), interleukin-4 (IL-4), interleukin-6
monoclonal antibodies (IL6), and tumor necrosis factor alpha (TNFα) was used
Fully developed and autologous bioprinted skin as per manufacturer instructions with the omission of
equivalents were washed twice with PBS before co-culture IL-8 detection antibodies. Plates were analyzed using a
with autologous PBMCs in 96-well plates with 1 × 10 QuickPlex multiplex plate reader (MSD). Supernatants
6
cells per well in Media E (Table 1). Skin equivalents were were analyzed in biological triplicates.
treated with 1 μg/mL of OKT3 (Jannsen-Cilag, UK) or 2.8. Statistical analysis
Tysabri (Biogen Idec Inc., US). Negative controls were Data are presented as mean ± standard deviation. Statistical
skin equivalents cultured in Media E with and without analyses for the quantified cytokine levels were conducted
autologous PBMCs and without biologics. The co-cultures using one-way analysis of variance (ANOVA) with Tukey
were incubated at standard culture conditions for 3 days HSD. Parameters for statistical significance were defined as
when 100 µL of supernatant was aspirated to analyze p ≤ 0.05 (*) and p ≤ 0.0001 (****).
proinflammatory cytokine release.
2.7. Multiplex proinflammatory cytokine 3. Results
detection assay 3.1. Printing parameter optimization and printing of
To quantify cytokine secretion in supernatants of skin cells
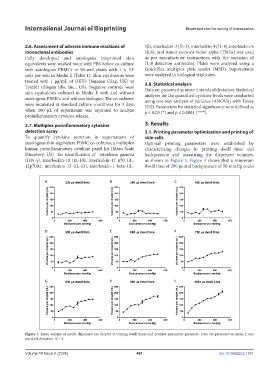
autologous skin-equivalent PBMC co-cultures, a multiplex Optimal printing parameters were established by
human proinflammatory cytokine panel kit (Meso Scale characterizing changes in printing dwell time and
Discovery, US) for identification of interferon gamma backpressure and measuring the dispensed volumes,
(IFN-γ), interleukin-10 (IL-10), interleukin-12 p70 (IL- as shown in Figure 3. Figure 3 shows that a minimum
12p70A), interleukin-13 (IL-13), interleukin-1 beta (IL- dwell time of 200 μs and backpressure of 50 mmHg could
Figure 3. Mean volume of media dispensed per droplet at varying dwell times and positive pneumatic pressures. Data are presented as mean ± one
standard deviation. N = 3.
Volume 10 Issue 2 (2024) 481 doi: 10.36922/ijb.1851