Page 491 - IJB-10-2
P. 491
International Journal of Bioprinting Bioprinted skin for testing of therapeutics
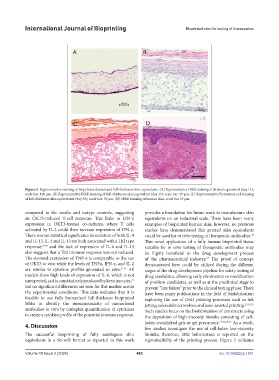
Figure 5. Representative staining of bioprinted dermal and full-thickness skin equivalents. (A) Representative H&E staining of dermal equivalent (day 14);
scale bar: 100 µm. (B) Representative H&E staining of full-thickness skin equivalent (day 35); scale bar: 50 µm. (C) Representative Picrosirius red staining
of full-thickness skin equivalents (day 35); scale bar: 50 µm. (D) H&E staining of human skin, scale bar 25 µm.
compared to the media and isotype controls, suggesting provides a foundation for future work to manufacture skin
an OKT3-induced T-cell response. This links to IFN-γ equivalents on an industrial scale. There have been many
expression in OKT3-treated co-cultures, where T cells examples of bioprinted human skin; however, no previous
activated by IL-2 could then increase expression of IFN-γ. studies have demonstrated that printed skin equivalents
There was no statistical significance in secretion of both IL-4 could be used for in vitro testing of therapeutic antibodies.
34
and IL-13. IL-4 and IL-13 are both associated with a Th2 type This novel application of a fully human bioprinted tissue
response, 29,30 and the lack of expression of IL-4 and IL-13 suitable for in vitro testing of therapeutic antibodies may
also suggests that a Th2 immune response was not induced. be highly beneficial to the drug development process
The elevated expression of TNF-α is comparable to the use of the pharmaceutical industry. The proof of concept
34
of OKT3 in vivo while the levels of TNFα, IFN-γ, and IL-2 demonstrated here could be utilized during the different
are similar to cytokine profiles generated in vitro. 31,32 All stages of the drug development pipeline for safety testing of
models show high levels of expression of IL-6, which is not drug candidates, allowing early elimination or modification
unexpected, as it is constitutively produced by keratinocytes, of problem candidates, as well as at the preclinical stage to
33
but no significant differences are seen for this marker across prevent “late failure” prior to the clinical testing phase. There
the experimental conditions. This data indicates that it is have been many publications in the field of biofabrication
feasible to use fully humanized full-thickness bioprinted exploring the use of DoD printing processes such as ink
HSEs to identify the immunotoxicity of monoclonal jetting, solenoid microvalves, and laser-assisted printing. 13,19,35
antibodies in vitro by multiplex quantification of cytokines Such studies focus on the biofabrication of constructs using
to create a cytokine profile of the potential immune response. the deposition of high-viscosity bioinks consisting of cell-
laden crosslinked gels or gel precursors. 14,20,36,37 As a result,
4. Discussion few studies investigate the use of cell-laden low-viscosity
The successful bioprinting of fully autologous skin bioinks; therefore, little information is reported on the
equivalents in a 96-well format as reported in this work reproducibility of the printing process. Figure 3 indicates
Volume 10 Issue 2 (2024) 483 doi: 10.36922/ijb.1851