Page 12 - IJB-3-1
P. 12
In vitro pre-vascularization strategies for tissue engineered constructs–Bioprinting and others
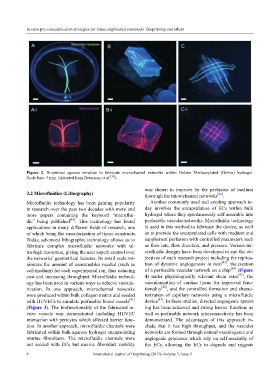
Figure 2. Bioprinted agarose template to fabricate microchannel networks within Gelatin Methacrylated (Gelma) hydrogel.
Scale bars: 3 mm. (Adopted from Betassoni et al. [39] )
was shown to improve by the perfusion of medium
3.2 Microfluidics (Lithography) thorough the microchannel networks [42] .
Microfluidic technology has been gaining popularity Another commonly used and exciting approach to-
in research over the past two decades with more and day involves the encapsulation of ECs within bulk
more papers containing the keyword “microflui- hydrogel where they spontaneously self-assemble into
dic” being published [40] . This technology has found perfusable vascular networks. Microfluidic technology
applications in many different fields of research, one is used in this method to fabricate the device, as well
of which being the vascularization of tissue constructs. as to provide the encapsulated cells with medium and
Today, advanced lithographic technology allows us to supplement perfusion with controlled parameters such
fabricate complex microfluidic networks with ul- as flow rate, flow direction, and pressure. Various mi-
tra-high resolution, giving the user superb control over crofluidic designs have been developed to suit the ob-
the networks’ geometrical features. Its small scale mi- jectives of each research project including the replica-
nimizes the amount of consumables needed (such as tion of dynamic angiogenesis in vitro [43] , the creation
cell medium) for each experimental run, thus reducing of a perfusable vascular network on a chip [44] (Figure
cost and increasing throughput. Microfluidic technol- 4) under physiologically relevant shear rates [45] , the
ogy has been used in various ways to achieve vascula- vascularization of cardiac tissue for improved func-
rization. In one approach, microchannel networks tionality [46] , and the controlled formation and charac-
were produced within bulk collagen matrix and seeded terization of capillary networks using a microfluidic
with HUVECs to simulate perfusable blood vessels [41] device [47] . In these studies, directed angiogenic sprout-
(Figure 3). The biofunctionality of the fabricated in ing has been achieved and strong barrier function, as
vitro vessels was demonstrated including HUVEC well as perfusable network interconnectivity has been
interaction with pericytes which affected barrier func- demonstrated. The advantages of this approach in-
tion. In another approach, microfluidic channels were clude that it has high throughput, and the vascular
fabricated within bulk agarose hydrogel encapsulating networks are formed through natural vasculogenic and
murine fibroblasts. The microfluidic channels were angiogenic processes which rely on self-assembly of
not seeded with ECs but murine fibroblast viability the ECs, allowing the ECs to degrade and migrate
8 International Journal of Bioprinting (2017)–Volume 3, Issue 1