Page 14 - IJB-3-1
P. 14
In vitro pre-vascularization strategies for tissue engineered constructs–Bioprinting and others
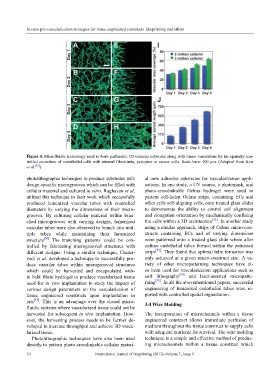
Figure 4. Microfluidic technology used to form perfusable 3D vascular networks along with tumor vasculature by the spatially con-
trolled co-culture of endothelial cells with stromal fibroblasts, pericytes or cancer cells. Scale bars: 100 μm. (Adopted from Kim
et al. [44] )
photolithographic techniques to produce substrates with al onto adhesive substrates for vascularization appli-
design-specific microgrooves which can be filled with cations. In one study, a UV source, a photomask, and
cellular material and cultured in vitro. Raghavan et al. photo-crosslinkable Gelma hydrogel were used to
utilized this technique in their work which successfully pattern cell-laden Gelma strips, containing ECs and
produced lumenized vascular tubes with controlled other cells self-aligning cells, onto treated glass slides
diameters by varying the dimensions of their micro- to demonstrate the ability to control cell alignment
grooves. By culturing cellular material within bran- and elongation orientation by mechanically confining
ched microgrooves with varying designs, lumenized the cells within a 3D architecture [52] . In another study
vascular tubes were also observed to branch into mul- using a similar approach, strips of Gelma micro-con-
tiple tubes while maintaining their lumenized structs containing ECs and of varying dimensions
structure [50] . The branching patterns could be con- were patterned onto a treated glass slide where after
trolled by fabricating microgrooved structures with culture endothelial tubes formed within the patterned
different designs. Using a similar technique, Chatur- strips [53] . They found that optimal tube formation was
vedi et al. developed a technique to successfully pro- only achieved at a given micro-construct size. A va-
duce vascular tubes within microgrooved structures riety of other micropatterning techniques have al-
which could be harvested and encapsulated with- so been used for vascularization applications such as
in bulk fibrin hydrogel to produce vascularized tissue soft lithography [54] and laser-assisted micropatte-
used for in vivo implantation to study the impact of rning [55] . In all the abovementioned papers, successful
various design parameters on the vascularization of engineering of lumenized endothelial tubes were re-
tissue engineered constructs upon implantation in ported with controlled spatial organization.
rats [51] . This is an advantage over the closed micro- 3.4 Wire Molding
fluidic systems where vascularized tissue could not be
harvested for subsequent in vivo implantation. How- The incorporation of microchannels within a tissue
ever, the harvesting process needs to be further de- engineered construct allows immediate perfusion of
veloped to increase throughput and achieve 3D vascu- medium throughout the tissue construct to supply cells
larized tissue. with adequate nutrients for survival. The wire molding
Photolithographic techniques have also been used technique is a simple and effective method of produc-
directly to pattern photo-crosslinkable cellular materi- ing microchannels within a tissue construct which
10 International Journal of Bioprinting (2017)–Volume 3, Issue 1