Page 16 - IJB-3-1
P. 16
In vitro pre-vascularization strategies for tissue engineered constructs–Bioprinting and others
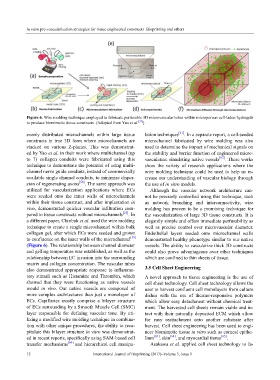
Figure 6. Wire molding technique employed to fabricate perfusable 3D microvascular tubes within microporous cell-laden hydrogels
to produce biomimetic tissue constructs. (Adopted from Yao et al. [57] )
evenly distributed microchannels within large tissue lation techniques [61] . In a separate report, a cell-seeded
constructs in true 3D form where microchannels are microchannel fabricated by wire molding was also
stacked on various Z-planes. This was demonstrat- used to determine the impact of mechanical signals on
ed by Yao et al. in their work where multichannel (up the stability and barrier function of engineered micro-
to 7) collagen conduits were fabricated using this vasculature simulating native vessels [62] . These works
technique to demonstrate the potential of using multi- show the variety of research applications where the
channel nerve guide conduits, instead of commercially wire molding technique could be used to help us in-
available single channel conduits, to minimize disper- crease our understanding of vascular biology through
sion of regenerating axons [58] . The same approach was the use of in vitro models.
utilized for vascularization applications where ECs Although the vascular network architecture can-
were seeded onto the inner walls of microchannels not be precisely controlled using this technique, such
within their tissue construct, and after implantation in as network branching and interconnectivity, wire
vivo, demonstrated quicker vascular infiltration com- molding has proven to be a promising technique for
pared to tissue constructs without microchannels [25] . In the vascularization of large 3D tissue constructs. It is
a different paper, Chrobak et al. used the wire molding elegantly simple and offers immediate perfusability as
technique to create a single microchannel within bulk well as precise control over microvascular diameter.
collagen gel, after which ECs were seeded and grown Endothelial layers seeded onto microchannel walls
to confluence on the inner walls of the microchannel [59] demonstrated healthy phenotype similar to our native
(Figure 6). The relationship between channel diameter vessels. The ability to vascularize thick 3D constructs
and gelling temperature was established, as well as the could also prove advantageous over other techniques
relationship between EC invasion into the surrounding which are confined to thin sheets of tissue.
matrix and collagen concentration. The vascular tubes
also demonstrated appropriate response to inflamma- 3.5 Cell Sheet Engineering
tory stimuli such as Histamine and Thrombin, which A novel approach to tissue engineering is the use of
showed that they were functioning as native vessels cell sheet technology. Cell sheet technology allows the
would in vivo. Our native vessels are composed of user to harvest confluent cell monolayers from culture
more complex architectures than just a monolayer of dishes with the use of thermo-responsive polymers
ECs. Capillaries mostly comprise a bilayer structure which allow easy detachment without chemical treat-
of ECs surrounding by a Smooth Muscle Cell (SMC) ment. The harvested cell sheets remain viable and in-
layer responsible for defining vascular tone. By uti- tact with their naturally deposited ECM which allow
lizing a modified wire molding technique in combina- for easy reattachment onto another substrate after
tion with other unique procedures, the ability to reca- harvest. Cell sheet engineering has been used to engi-
pitulate this bilayer structure in vitro was demonstrat- neer biomimetic tissue in vitro such as corneal epithe-
ed in recent reports, specifically using SAM-based cell lium [63] , skin [64] , and myocardial tissue [65] .
transfer mechanisms [60] and hierarchical cell manipu- Asakawa et al. applied cell sheet technology to fa-
12 International Journal of Bioprinting (2017)–Volume 3, Issue 1