Page 322 - IJB-10-3
P. 322
International Journal of Bioprinting 3D-bioprinted hydrogel for pulp regeneration
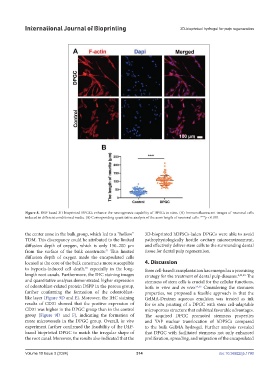
Figure 8. DLP-based 3D-bioprinted DPGCs enhance the neurogenesis capability of DPSCs in vitro. (A) Immunofluorescent images of neuronal cells
induced in different conditioned media. (B) Corresponding quantitative analysis of the axon length of neuronal cells. ***p < 0.001.
the center zone in the bulk group, which led to a “hollow” 3D-bioprinted hDPSCs-laden DPGCs were able to avoid
TDM. This discrepancy could be attributed to the limited pathophysiologically hostile cavitary microenvironment,
diffusion depth of oxygen, which is only 150–200 μm and effectively deliver stem cells to the surrounding dental
51
from the surface of the bulk constructs. This limited tissue for dental pulp regeneration.
diffusion depth of oxygen made the encapsulated cells
located at the core of the bulk constructs more susceptible 4. Discussion
18
to hypoxia-induced cell death, especially in the long- Stem cell-based transplantation has emerged as a promising
length root canals. Furthermore, the IHC staining images strategy for the treatment of dental pulp diseases. 8,52,53 The
and quantitative analyses demonstrated higher expression stemness of stem cells is crucial for the cellular functions,
of odontoblast-related protein DSPP in the porous group, both in vitro and in vivo. 54,55 Considering the stemness
further confirming the formation of the odontoblast- properties, we proposed a feasible approach in that the
like layer (Figure 9D and E). Moreover, the IHC staining GelMA-Dextran aqueous emulsion was treated as ink
results of CD31 showed that the positive expression of for in situ printing of a DPGC with stem cell-adaptable
CD31 was higher in the DPGC group than in the control microporous structure that exhibited favorable advantages.
group (Figure 9D and F), indicating the formation of The acquired DPGC promoted stemness properties
more microvessels in the DPGC group. Overall, in vivo and YAP nuclear translocation of hDPSCs compared
experiment further confirmed the feasibility of the DLP- to the bulk GelMA hydrogel. Further analysis revealed
based bioprinted DPGC to match the irregular shape of that DPGC with facilitated stemness not only enhanced
the root canal. Moreover, the results also indicated that the proliferation, spreading, and migration of the encapsulated
Volume 10 Issue 3 (2024) 314 doi: 10.36922/ijb.1790