Page 318 - IJB-10-3
P. 318
International Journal of Bioprinting 3D-bioprinted hydrogel for pulp regeneration
phase of odontoblast differentiation. Figure 4A shows that 3.3. Biological properties of 3D-bioprinted DPGC
substantial increases in ALP activity were noted both in the for promoting in vitro pulp regeneration potential
DPGC and control groups after 7–14 days of culture. There of DPSCs
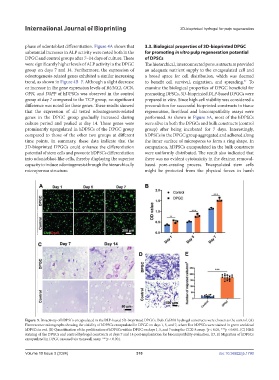
were significantly higher levels of ALP activity in the DPGC The hierarchical, interconnected porous structure provided
group on days 7 and 14. Furthermore, the expression of an adequate nutrient supply to the encapsulated cell and
odontogenesis-related genes exhibited a similar increasing a broad space for cell distribution, which was deemed
trend, as shown in Figure 4B–F. Although a slight decrease to benefit cell survival, migration, and spreading. To
41
or increase in the gene expression levels of RUNX2, OCN, examine the biological properties of DPGC beneficial for
OPN, and DSPP of hDPSCs was observed in the control promoting DPSCs, 3D-bioprinted DLP-based DPGCs were
group at day 7 compared to the TCP group, no significant prepared in vitro. Since high cell viability was considered a
difference was noted for these genes. These results showed precondition for successful bioprinted constructs in tissue
that the expression of all tested odontogenesis-related regeneration, live/dead and biocompatibility assays were
genes in the DPGC group gradually increased during performed. As shown in Figure 5A, most of the hDPSCs
culture period and peaked at day 14. These genes were were alive in both the DPGCs and bulk constructs (control
prominently upregulated in hDPSCs of the DPGC group group) after being incubated for 7 days. Interestingly,
compared to those of the other two groups at different hDPSCs in the DPGC group aggregated and adhered along
time points. In summary, these data indicate that the the inner surface of micropores to form a ring shape. In
3D-bioprinted DPGCs could enhance the differentiation comparison, hDPSCs encapsulated in the bulk constructs
potential of stem cells and promote hDPSCs differentiation were uniformly distributed. The result also indicated that
into odontoblast-like cells, thereby displaying the superior there was no evident cytotoxicity in the dextran removal-
capacity to induce odontogenesis through the hierarchically based pore-creating process. Encapsulated stem cells
microporous structure. might be protected from the physical forces in harsh
Figure. 5. Bioactivity of hDPSCs encapsulated in the DLP-based 3D-bioprinted DPGCs. Bulk GelMA hydrogel constructs were chosen as the control. (A)
Fluorescence micrographs showing the viability of hDPSCs encapsulated in DPGC on days 1, 5, and 7, where live hDPSCs were stained in green and dead
hDPSCs in red. (B) Quantification of the proliferation of hDPSCs within DPGC on days 1, 5, and 7 using the CCK-8 assay. *p < 0.05, ***p < 0.001. (C) H&E
staining of the DPGCs and control hydrogel constructs at days 7 and 14 post-implantation for biocompatibility evaluation. (D, E) Migration of hDPSCs
encapsulated in DPGC assessed via transwell assay. ***p < 0.001.
Volume 10 Issue 3 (2024) 310 doi: 10.36922/ijb.1790