Page 422 - IJB-10-3
P. 422
International Journal of Bioprinting Expanding 3D cell proliferation with DLP bioprinting
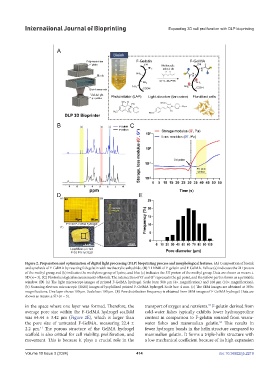
Figure 2. Preparation and optimization of digital light processing (DLP) bioprinting process and morphological features. (A) Composition of bioink
and synthesis of F-GelMA by reacting fish gelatin with methacrylic anhydride. (B) H NMR of F-gelatin and F-GelMA. Yellow (a) indicates the 2H proton
1
of the methyl group, red (b) indicates the methylene group of lysine, and blue (c) indicates the 3H proton of the methyl group. Data are shown as means ±
SD (n = 3). (C) Photorheological measurements of bioink. The intersection of G’ and G’’ represents the gel point, and the yellow part is shown as a printable
window. (D) (a) The light microscope images of printed F-GelMA hydrogel. Scale bars: 500 µm (4× magnification) and 100 µm (10× magnification).
(b) Scanning electron microscopic (SEM) images of lyophilized printed F-GelMA hydrogel. Scale bar: 4 mm. (c) The SEM images are obtained at 300×
magnifications. One layer shows 100 µm. Scale bar: 100 µm. (E) Pore distribution frequency is obtained from SEM images of F-GelMA hydrogel. Data are
shown as means ± SD (n = 3).
in the space where one layer was formed. Therefore, the transport of oxygen and nutrients. F-gelatin derived from
57
average pore size within the F-GelMA hydrogel scaffold cold-water fishes typically exhibits lower hydroxyproline
was 64.44 ± 3.42 μm (Figure 2E), which is larger than content in comparison to F-gelatin sourced from warm-
the pore size of untreated F-GelMA, measuring 22.4 ± water fishes and mammalian gelatin. This results in
58
2.2 μm. The porous structure of the GelMA hydrogel fewer hydrogen bonds in the helix structure compared to
41
scaffold is also critical for cell viability, proliferation, and mammalian gelatin. It forms a triple-helix structure with
movement. This is because it plays a crucial role in the a low mechanical coefficient because of its high expansion
Volume 10 Issue 3 (2024) 414 doi: 10.36922/ijb.2219