Page 426 - IJB-10-3
P. 426
International Journal of Bioprinting Expanding 3D cell proliferation with DLP bioprinting
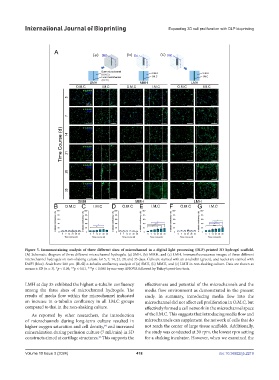
Figure 5. Immunostaining analysis of three different sizes of microchannel in a digital light processing (DLP)-printed 3D hydrogel scaffold.
(A) Schematic diagram of three different microchannel hydrogels: (a) SMH, (b) MMH, and (c) LMH. Immunofluorescence images of three different
microchannel hydrogels on non-shaking culture for 5, 7, 14, 21, 28, and 35 days. Cells are stained with an α-tubulin (green), and nuclei are stained with
DAPI (blue). Scale bars: 200 µm. (B–G) α-tubulin confluency analysis of (a) SMH, (b) MMH, and (c) LMH in non-shaking culture. Data are shown as
means ± SD (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001 by one-way ANOVA followed by Tukey’s post-hoc tests.
LMH at day 35 exhibited the highest α-tubulin confluency effectiveness and potential of the microchannels and the
among the three sizes of microchannel hydrogels. The media flow environment as demonstrated in the present
results of media flow within the microchannel indicated study. In summary, introducing media flow into the
an increase in α-tubulin confluency in all I.M.C groups microchannel did not affect cell proliferation in O.M.C, but
compared to that in the non-shaking culture. effectively formed a cell network in the microchannel space
As reported by other researchers, the introduction of the I.M.C. This suggests that introducing media flow and
of microchannels during long-term culture resulted in microchannels can supplement the network of cells that do
higher oxygen saturation and cell density, and increased not reach the center of large tissue scaffolds. Additionally,
64
mineralization during perfusion culture (7 mL/min) in 3D the study was conducted at 30 rpm, the lowest rpm setting
constructs aimed at cartilage structures. This supports the for a shaking incubator. However, when we examined the
26
Volume 10 Issue 3 (2024) 418 doi: 10.36922/ijb.2219