Page 85 - IJB-4-2
P. 85
Shuai C et al.
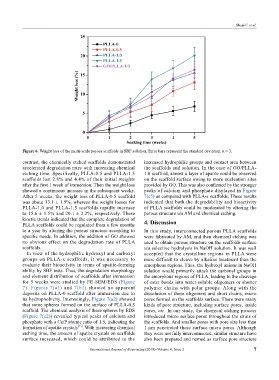
Figure 6. Weight loss of the multi-scale porous scaffolds in SBF solution. Error bars represent the standard deviation. n = 3.
contrast, the chemically etched scaffolds demonstrated increased hydrophilic groups and contact area between
accelerated degradation rates with increasing chemical the scaffolds and solution. In the case of GO/PLLA-
etching time. Specifically, PLLA-0.5 and PLLA-1.5 1.0 scaffold, almost a layer of apatite could be observed
scaffolds lost 2.8% and 4.4% of their initial weights on the scaffold surface owing to more nucleation sites
after the first 1 week of immersion. Then the weight loss provided by GO. This was also confirmed by the stronger
showed a continuous increase in the subsequent weeks. peaks of calcium and phosphate displayed in Figure
After 5 weeks, the weight loss of PLLA-0.5 scaffold 7(c5) as compared with PLLA-x scaffolds. These results
was about 13.1 ± 1.9%, whereas the weight losses for indicated that both the degradability and bioactivity
PLLA-1.0 and PLLA-1.5 scaffolds rapidly increase of PLLA scaffolds could be moderated by altering the
to 15.6 ± 1.5% and 20.1 ± 2.2%, respectively. These porous structure via AM and chemical etching.
kinetic trends indicated that the complete degradation of 4. Discussion
PLLA scaffolds could be regulated from a few months
to a year by altering the porous structure according to In this study, interconnected porous PLLA scaffolds
specific needs. In addition, the addition of GO showed were fabricated by AM, and then chemical etching was
no obvious effect on the degradation rate of PLLA used to obtain porous structure on the scaffolds surface
scaffolds. via selective hydrolysis in NaOH solution. It was well
In view of the hydrophilic hydroxyl and carboxyl accepted that the crystalline regions in PLLA were
groups on PLLA-x scaffolds, it was necessary to more difficult to cleave by alkaline treatment than the
evaluate their bioactivity in terms of apatite-forming amorphous regions. Thus, the hydroxyl anions in NaOH
ability by SBF tests. Thus, the degradation morphology solution would primarily attack the carbonyl groups in
and element distribution of scaffolds after immersion the amorphous regions of PLLA, leading to the cleavage
for 5 weeks were studied by FE-SEM/EDS (Figure of ester bonds into water-soluble oligomers or shorter
7). Figures 7(a1) and 7(b1) showed no apparent polymer chains with polar groups. Along with the
deposits on PLLA-0 scaffold after immersion due to dissolution of these oligomers and short chains, micro
its hydrophobicity. Interestingly, Figure 7(a2) showed pores formed on the scaffolds surface. There were many
that some spheres formed on the surface of PLLA-0.5 kinds of pore structure, including surface pores, inside
scaffold. The chemical analysis of these spheres by EDS pores, etc. In our study, the chemical etching process
(Figure 7(c2)) revealed typical peaks of calcium and introduced micro surface pores throughout the struts of
phosphate with a Ca/P atomic ratio of 1.5, indicating the the scaffolds. And smaller pores with pore size less than
[33]
formation of apatite crystals . With increasing chemical 1 μm penetrated these surface micro pores. Although
etching time, the amount of apatite crystals on scaffolds they were not fully interconnected, similar structure have
surface increased, which could be attributed to the also been prepared and named as surface pore structure
International Journal of Bioprinting (2018)–Volume 4, Issue 2 7