Page 218 - IJB-10-4
P. 218
International Journal of Bioprinting 3D-Printed scaffolds for diabetic bone defects
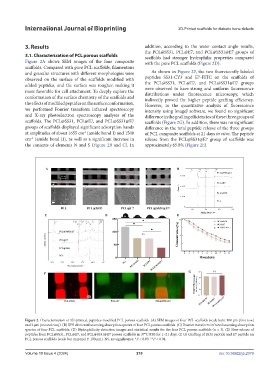
3. Results addition, according to the water contact angle results,
the PCL@SS31, PCL@E7, and PCL@SS31@E7 groups of
3.1. Characterization of PCL porous scaffolds scaffolds had stronger hydrophilic properties compared
Figure 2A shows SEM images of the four composite with the pure PCL scaffolds (Figure 2D).
scaffolds. Compared with pure PCL scaffolds, filamentous
and granular structures with different morphologies were As shown in Figure 2F, the two fluorescently labeled
observed on the surface of the scaffolds modified with peptides SS31-CY3 and E7-FITC on the scaffolds of
added peptides, and the surface was rougher, making it the PCL@SS31, PCL@E7, and PCL@SS31@E7 groups
more favorable for cell attachment. To deeply explore the were observed to have strong and uniform fluorescence
distributions under fluorescence microscopy, which
conformation of the surface chemistry of the scaffolds and indirectly proved the higher peptide grafting efficiency.
the effects of modified peptides on the surface conformation, However, in the quantitative analysis of fluorescence
we performed Fourier transform infrared spectroscopy intensity using ImageJ software, we found no significant
and X-ray photoelectron spectroscopy analyses of the difference in the grafting efficiencies of these three groups of
scaffolds. The PCL@SS31, PCL@E7, and PCL@SS31@E7 scaffolds (Figure 2G). In addition, there was no significant
groups of scaffolds displayed significant adsorption bands difference in the total peptide release of the three groups
at amplitudes of about 1655 cm (amide bond I) and 1508 of PCL composite scaffolds at 21 days in vitro. The peptide
-1
cm (amide bond II), as well as a significant increase in release from the PCL@SS31@E7 group of scaffolds was
-1
the contents of elements N and S (Figure 2B and C). In approximately 65.8% (Figure 2E).
Figure 2. Characterization of 3D-printed, peptides-modified PCL porous scaffolds. (A) SEM images of four PCL scaffolds (scale bars: 100 μm [first row]
and 2 μm [second row]). (B) XPS elemental scanning absorption spectra of four PCL porous scaffolds. (C) Fourier transform infrared scanning absorption
spectra of four PCL scaffolds. (D) Hydrophilicity detection images and statistical results for the four PCL porous scaffolds (n = 3). (E) Slow release of
peptides from PCL@SS31, PCL@E7, and PCL@SS31@E7 porous scaffolds in 37°C PBS for 1–21 days. (F, G) Grafting of SS31 peptide and E7 peptide on
PCL porous scaffolds (scale bar on panel F: 100 μm). NS, no significance; *P < 0.05; **P < 0.01.
Volume 10 Issue 4 (2024) 210 doi: 10.36922/ijb.2379