Page 223 - IJB-10-4
P. 223
International Journal of Bioprinting 3D-Printed scaffolds for diabetic bone defects
that 75 genes were jointly upregulated in the PCL@SS31, E7 porous scaffolds to promote osteogenic differentiation
PCL@E7, and PCL@SS31@E7 groups, as compared to the of BMSCs. Based on these findings, we postulate a
PCL group (Figure 5F and G); in the Kyoto Encyclopedia mechanism by which PCL@SS31@E7 porous scaffolds
of Genes and Genomes pathway enrichment of these 75 improve mitochondrial function, i.e., a high-glucose
genes, the “MAPK signaling pathway,” “mineral absorption,” environment causes mitochondrial electron leakage and
“Hedgehog signaling pathway,” and “Wnt signaling pathway” impaired cellular osteogenic function, and the SS31 peptide
were ranked at positions 2, 12, 13, and 16, respectively from the scaffold restores cellular osteogenic function
(Figure 5H). Gene ontology cell function enrichment by reducing mitochondrial electron leakage, increasing
analysis revealed significant enrichment of these genes in glucose consumption, and promoting ATP production and
“blood vessel morphogenesis/development,” “regulation osteogenic differentiation in BMSCs (Figure 5J).
of bone remodeling,” “bone morphogenesis,” and “bone 3.4. Regeneration of bone defects in diabetic rats
development” (Figure 5I). All these results suggest that the Two-month-old male SD rats were selected and raised
MAPK/Hedgehog/Wnt signaling pathway may be related to with a high-glucose and high-fat diet for 1 month and
the promotion of the osteogenic differentiation of BMSCs subcutaneously injected with STZ, and blood glucose
under high-glucose conditions in the PCL@SS31@E7 group. measurements were taken for each rat after 2 weeks. Blood
The results also further confirm the ability of PCL@SS31@ glucose values ≥11.1 mmol/L indicated that the diabetic
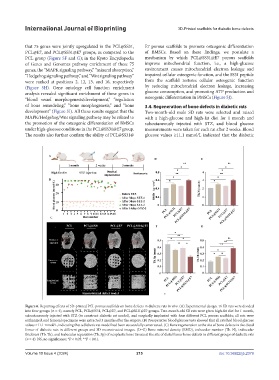
Figure 6. Repairing effects of 3D-printed PCL porous scaffolds on bone defects in diabetic rats in vivo. (A) Experimental design: 16 SD rats were divided
into four groups (n = 4), namely PCL, PCL@SS31, PCL@E7, and PCL@SS31@E7 groups. Two-month-old SD rats were given high-fat diet for 1 month,
subcutaneously injected with STZ (to construct diabetic rat model), and surgically implanted with four different PCL porous scaffolds; all rats were
euthanized, and femoral specimens were extracted 3 months after the surgery. (B) Preoperative blood glucose tests showed that all rats had blood glucose
values ≥11.1 mmol/L, indicating that a diabetic rat model had been successfully constructed. (C) Bone regeneration at the site of bone defects in the distal
femur of diabetic rats in different groups and 3D reconstructed images. (D–G) Bone mineral density (BMD), trabecular number (Tb. N), trabecular
thickness (Tb. Th), and trabecular separation (Tb. Sp) of neoplastic bone tissues at the site of distal femur bone defects in different groups of diabetic rats
(n = 4). NS, no significance; *P < 0.05; **P < 0.01.
Volume 10 Issue 4 (2024) 215 doi: 10.36922/ijb.2379