Page 236 - IJB-10-4
P. 236
International Journal of Bioprinting N-PLN hydrogels for human skin modeling
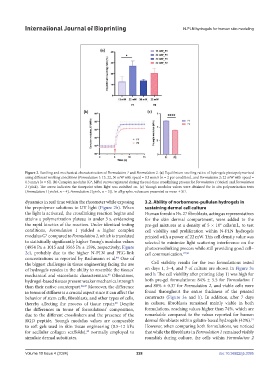
Figure 2. Swelling and mechanical characterization of Formulation 1 and Formulation 2. (a) Equilibrium swelling ratios of hydrogels photopolymerized
using different working conditions (Formulation 1: 13, 22, 30 mW with speed = 0.3 mm/s [n = 2 per condition], and Formulation 2: 22 mW with speed =
0.3 mm/s [n = 6]). (b) Complex modulus (G*, MPa) curves registered during the real-time crosslinking process for Formulation 1 (violet) and Formulation
2 (pink). The arrow indicates the timepoint when light was switched on. (c) Young’s modulus values were obtained for in situ polymerization tests
(Formulation 1 [violet, n = 4], Formulation 2 [pink, n = 5]). In all graphs, values are presented as mean ± SD.
dynamics in real time within the rheometer while exposing 3.2. Ability of norbornene-pullulan hydrogels in
the prepolymer solutions to UV light (Figure 2b). When sustaining dermal cell culture
the light is activated, the crosslinking reaction begins and Human foreskin Hs-27 fibroblasts, acting as representatives
attains a polymerization plateau in under 3 s, evidencing for the skin dermal compartment, were added to the
the rapid kinetics of the reaction. Under identical testing pre-gel mixtures at a density of 5 × 10 cells/mL, to test
6
conditions, Formulation 1 yielded a higher complex cell viability and proliferation within N-PLN hydrogels
modulus G* compared to Formulation 2, which is translated printed with a power of 22 mW. This cell density value was
to statistically significantly higher Young’s modulus values selected to minimize light scattering interference on the
(4954 Pa ± 1015 and 3565 Pa ± 1596, respectively; Figure photocrosslinking process while still providing good cell–
2c), probably due to the higher N-PLN and PEG-link cell communication. 27,50
concentrations as reported by Bachmann et al. One of
44
the biggest challenges in tissue engineering facing the use Cell viability results for the two formulations tested
of hydrogels resides in the ability to resemble the tissues’ on days 1, 3–4, and 7 of culture are shown in Figure 3a
mechanical and viscoelastic characteristics. Oftentimes, and b. The cell viability after printing (day 1) was high for
45
hydrogel-based tissues present weaker mechanical strength both pre-gel formulations: 84% ± 5.5 for Formulation 1
than their native counterpart. 46,47 Moreover, the difference and 88% ± 0.37 for Formulation 2, and viable cells were
in terms of stiffness is a crucial aspect since it can affect the found throughout the entire thickness of the printed
behavior of stem cells, fibroblasts, and other types of cells, constructs (Figure 3a and b). In addition, after 7 days
thereby affecting the process of tissue repair. Despite in culture, fibroblasts remained mainly viable in both
48
the differences in terms of formulations’ composition, formulations, reaching values higher than 74%, which are
due to the different crosslinkers and the presence of the remarkable compared to the values reported for human
RGD peptide, Young’s modulus values are comparable dermal fibroblasts within gelatin-based hydrogels (42%).
51
to soft gels used in skin tissue engineering (0.5–12 kPa However, when comparing both formulations, we noticed
for acellular collagen scaffolds), normally employed to that while the fibroblasts in Formulation 1 remained visibly
49
simulate dermal substitutes. roundish during culture, the cells within Formulation 2
Volume 10 Issue 4 (2024) 228 doi: 10.36922/ijb.3395