Page 238 - IJB-10-4
P. 238
International Journal of Bioprinting N-PLN hydrogels for human skin modeling
cells and stimulating their growth, due to the presence of a with a combination of different types of cell culture
natural-based crosslinker (HA) and RGD peptide. medium both in the apical or basolateral compartments or
even in the absence of medium in the apical compartment.
3.3. Epithelized dermal skin constructs: Toward full-
thickness skin In our model, the seeding time point was established
After confirming the suitability of the hydrogels from at 3 days post-fibroblast encapsulation, to achieve a
Formulation 2 to create a dermal compartment, a co- proper fibroblast elongation within the matrix so as
culture model was developed to produce an epithelized to sustain the epithelial culture. The same medium
dermal skin construct. Hence, HaCaT cells, which are was added either into the apical and the basolateral
extensively used in cell culture studies, wound healing, compartments, keeping the samples fully submerged for 7
and transplantations, 54–56 were seeded on top of the dermal days (submerged condition). Afterward, ALI conditions,
constructs to mimic the epidermal compartment. To which are necessary to induce keratinocyte differentiation
58
allow for the presence of distinctive apical and basolateral and stratification, were applied to half of the samples,
compartments, the constructs were mounted in Transwell® meaning that the medium was removed from the apical
inserts. HaCaT cells are commonly maintained in normal compartment and only added into the basolateral one,
DMEM supplemented with 10% FBS because both contain whereas the other half was kept in submerged conditions.
sufficient calcium to sustain their growth and to induce Then, all samples were maintained in co-culture and
differentiation, which can also be triggered by the monitored for an additional 2 weeks (up to 21 days post-
57
application of specific culture conditions, such as culturing HaCaT seeding) (Figure 4a).
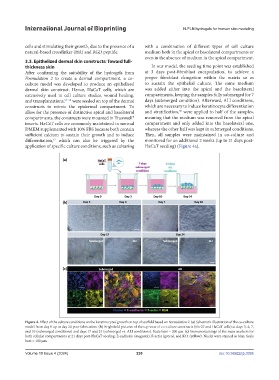
Figure 4. Effect of the culture conditions on the keratinocytes’ growth on top of scaffold based on Formulation 2. (a) Schematic illustration of the co-culture
model from day 0 up to day 24 post-fabrication. (b) Brightfield pictures of the top view of co-culture constructs (Hs-27 and HaCaT cells) at days 3, 4, 7,
and 10 (submerged conditions) and days 17 and 24 (submerged vs. ALI conditions). Scale bars = 200 µm. (c) Immunostainings of the main markers for
both cellular compartments at 21 days post-HaCaT seeding: E-cadherin (magenta), F-actin (green), and K14 (yellow). Nuclei were stained in blue. Scale
bars = 100 µm.
Volume 10 Issue 4 (2024) 230 doi: 10.36922/ijb.3395