Page 239 - IJB-10-4
P. 239
International Journal of Bioprinting N-PLN hydrogels for human skin modeling
Brightfield inspection of the samples revealed cells and better distinguish the multilayered distribution of the
fully covering the fibroblast-laden hydrogels by day 10 keratinocytes. E-cadherin signal appeared clustered on the
(Figure 4b). By days 17 and 24 of the experiment, images scaffolds’ surface, especially in the submerged condition,
did not reveal significant differences in terms of cellular where some uncovered portions were also observed
morphology between the submerged and ALI culture (left panel of Figure 4c). A more uniform and consistent
conditions. In both cases, regions with some clustered cells distribution of E-cadherin was found on top of hydrogels
could be distinguished, which might be related to HaCaT cultured in ALI conditions (right panel of Figure 4c),
multilayer formation. To elucidate that, the samples were corroborating the importance of the ALI for the growth of
analyzed by immunofluorescence microscopy at day 24 of human keratinocytes.
the experiment (21 days post-HaCaT seeding). Figure 4c Next, the cellular organization and functionality of
shows the dermal fibroblasts (here visualized by the staining both skin dermal and epidermal compartments were
of F-actin), which forms a network that appears more investigated upon the expression of specific markers for
compact for the samples cultured in ALI conditions. As both submerged and ALI conditions. Figure 5a shows
specific markers for the keratinocytes, both keratin 14 and representative cross-sections of the resulting hydrogels,
E-cadherin were selected (stained in yellow and magenta, where vimentin was stained to highlight the dermal
respectively) (Figure 4c). Keratin 14 antibody was used to compartment, E-cadherin was chosen as an epidermal
mark the basal layer of non-differentiated epidermis. The marker, and laminin was selected as an extracellular matrix
keratin 14 signal was visible for samples cultured in both protein marker. Both dermal and epidermal compartments
submerged and ALI conditions. However, morphological were visibly segregated within the samples. Fibroblasts
differences between the submerged and ALI conditions appeared elongated and uniformly distributed within the
could be observed, with the latter featuring a more evenly bulk of the hydrogels, forming a network, and laying below
distributed signal of keratin 14 (right panel in Figure 4c). the epithelial cells for both culture conditions. In addition,
Comparable results were found with the E-cadherin signal. the fibroblasts under the ALI condition formed a more
This marker was visualized to examine cell–cell contacts entangled mesh as compared to the submerged ones.
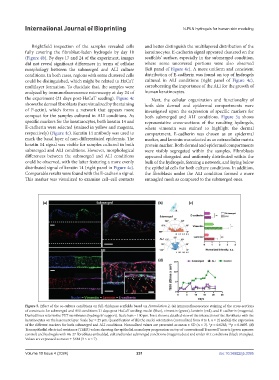
Figure 5. Effect of the co-culture conditions on full-thickness scaffolds based on Formulation 2. (a) Immunofluorescence staining of the cross-sections
of constructs for submerged and ALI conditions 21 days post-HaCaT seeding: nuclei (blue), vimentin (green), laminin (red), and E-cadherin (magenta).
Dashed lines refer to the PET membranes (hydrogels’ support). Scale bars = 100 µm. Inset shows a detailed view of the interaction of the fibroblasts with the
keratinocytes on the basement layer. Scale bar = 25 µm. Quantification of (b) the nuclei orientation (normalized from 0 to 1, n = 2) and (c) the expression
of the different markers for both submerged and ALI conditions. Normalized values are presented as mean ± SD (n = 3). *p = 0.0283; **p = 0.0495. (d)
Transepithelial electrical resistance (TEER) values showing the epithelial monolayer progression on top of conventional Transwell inserts (green squares:
®
control) and hydrogels with Hs-27 fibroblasts embedded, cultured under submerged conditions (magenta dots) and under ALI conditions (black triangles).
Values are expressed as mean ± SEM (3 ≤ n ≤ 7).
Volume 10 Issue 4 (2024) 231 doi: 10.36922/ijb.3395