Page 357 - IJB-10-4
P. 357
International Journal of Bioprinting Stiffness of scaffold-mediated immune response
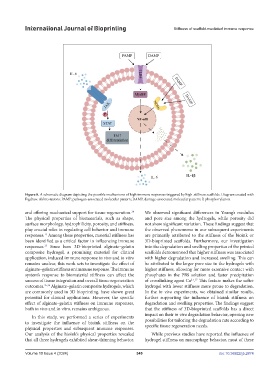
Figure 8. A schematic diagram depicting the possible mechanisms of high immune responses triggered by high-stiffness scaffolds. Diagram created with
Figdraw. Abbreviations: PAMP, pathogen-associated molecular pattern; DAMP, damage-associated molecular pattern; P, phosphorylation.
and offering mechanical support for tissue regeneration. We observed significant differences in Young’s modulus
34
The physical properties of biomaterials, such as shape, and pore size among the hydrogels, while porosity did
surface morphology, hydrophilicity, porosity, and stiffness, not show significant variation. These findings suggest that
play crucial roles in regulating cell behavior and immune the observed phenomena in our subsequent experiments
responses. Among these properties, material stiffness has are primarily attributed to the stiffness of the bioink or
13
been identified as a critical factor in influencing immune 3D-bioprinted scaffolds. Furthermore, our investigation
responses. Since how 3D-bioprinted alginate–gelatin into the degradation and swelling properties of the printed
35
composite hydrogel, a promising material for clinical scaffolds demonstrated that higher stiffness was associated
application, induced immune response in vivo and in vitro with higher degradation and increased swelling. This can
remains unclear, this work sets to investigate the effect of be attributed to the larger pore size in the hydrogels with
alginate–gelatin stiffness on immune response. The immune higher stiffness, allowing for more extensive contact with
system’s response to biomaterial stiffness can affect the phosphates in the PBS solution and faster precipitation
2+ 37
success of tissue integration and overall tissue regeneration of crosslinking agent Ca . This feature makes the softer
outcomes. 35,36 Alginate–gelatin composite hydrogels, which hydrogel with lower stiffness more prone to degradation.
are commonly used in 3D bioprinting, have shown great In the in vivo experiments, we obtained similar results,
potential for clinical applications. However, the specific further supporting the influence of bioink stiffness on
effect of alginate–gelatin stiffness on immune responses, degradation and swelling properties. The findings suggest
both in vivo and in vitro, remains ambiguous. that the stiffness of 3D-bioprinted scaffolds has a direct
In this study, we performed a series of experiments impact on their in vivo degradation behavior, opening new
to investigate the influence of bioink stiffness on the possibilities for tailoring the degradation rate according to
physical properties and subsequent immune responses. specific tissue regeneration needs.
Our analysis of the bioink’s physical properties revealed While previous studies have reported the influence of
that all three hydrogels exhibited shear-thinning behavior. hydrogel stiffness on macrophage behavior, most of these
Volume 10 Issue 4 (2024) 349 doi: 10.36922/ijb.2874