Page 46 - IJB-10-5
P. 46
International Journal of Bioprinting Bioprinted tumor immune microenvironment
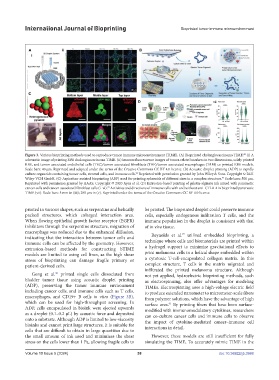
Figure 3. Various bioprinting methods used to reproduce tumor immune microenvironment (TIME). (A) Bioprinted cholangiocarcinoma TIME. (i) A
88
schematic image of printing RBE cholangiocarcinoma TIME. (ii) Immunofluorescence images of tumor-related markers in two dimensions, solely printed
RBE, and tumor-associated endothelial cells (TEC)/tumor-associated fibroblasts (TAF)/tumor-associated macrophages (TAM) co-printed RBE models.
Scale bars: 40 µm. Reprinted and adapted under the terms of the Creative Commons CC BY 4.0 license. (B) Acoustic droplet printing (ADP) to rapidly
culture organoids containing tumor cells, stromal cells, and immune cells. Reprinted with permission granted by John Wiley & Sons. Copyright © 2021
90
Wiley-VCH GmbH. (C) Aspiration-assisted bioprinting (ABP) used for printing spheroids of different sizes in a complex structure. Scale bars: 500 µm.
94
Reprinted with permission granted by AAAS. Copyright © 2020 Ayan et al. (D) Extrusion-based printing of gelatin-alginate ink mixed with pancreatic
cancer cells and cancer-associated fibroblast cells (i–vi). Activities and directions of immune cells with and without anti-CTLA-4 in bioprinted pancreatic
79
TIME (vii). Scale bars: 5 mm in (iii); 200 µm in (v). Reprinted under the terms of the Creative Commons CC BY 4.0 license.
printed in various shapes, such as serpentine and helically be printed. The bioprinted droplet could preserve immune
packed structures, which enlarged interaction area. cells, especially endogenous infiltration T cells, and the
When flowing epithelial growth factor receptor (EGFR) immune population in the droplet is consistent with that
inhibitors through the serpentine structure, migration of of in vivo tissue.
macrophage was reduced due to the enhanced diffusion, 77
indicating that the interaction between tumor cells and Reynolds et al. utilized embedded bioprinting, a
immune cells can be affected by the geometry. However, technique where cells and biomaterials are printed within
extrusion-based methods for constructing bTIME a hydrogel support to minimize gravitational effects to
models are limited to using cell lines, as the high shear print melanoma cells in a helical shape embedded within
stress of bioprinting can damage fragile primary or a cytotoxic T-cell-encapsulated collagen matrix. In this
patient-derived cells. complex structure, T cells in the matrix migrated and
infiltrated the printed melanoma structure. Although
Gong et al. printed single cells dissociated from not yet applied, hydroelectric bioprinting methods, such
90
bladder tumor tissue using acoustic droplet printing as electrospinning, also offer advantages for modeling
(ADP), preserving the tumor immune environment TIMEs. Electrospinning uses a high-voltage electric field
including cancer cells, and immune cells such as T cells, to produce extended nanometer to micrometer-scale fibers
macrophages, and CD19+ B cells in vitro (Figure 3B), from polymer solutions, which have the advantage of high
which can be used for high-throughput screening. In surface area. By printing fibers that have been surface-
91
ADP, cells encapsulated in bioink were ejected upwards modified with immunomodulatory cytokines, researchers
as a droplet (0.1–0.2 μL) by acoustic force and deposited can co-culture cancer cells and immune cells to observe
onto a substrate. Although ADP is limited to low-viscosity the impact of cytokine-mediated cancer–immune cell
bioinks and cannot print large structures, it is suitable for interactions in detail.
cells that are difficult to obtain in large quantities due to
the small amount of ink used and minimizes the shear However, these models are still insufficient for fully
stress on the cells lower than 1 Pa, allowing fragile cells to simulating the TIME. To accurately mimic TIME in the
Volume 10 Issue 5 (2024) 38 doi: 10.36922/ijb.3988