Page 110 - IJB-6-2
P. 110
Scaffolds produced by combining porogen leaching and emulsion templating
A B C
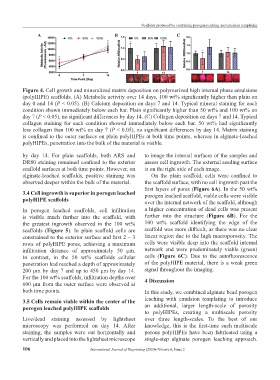
Figure 4. Cell growth and mineralized matrix deposition on polymerized high internal phase emulsions
(polyHIPE) scaffolds. (A) Metabolic activity over 14 days, 100 wt% significantly higher than plain on
day 0 and 14 (P < 0.05). (B) Calcium deposition on days 7 and 14. Typical mineral staining for each
condition shown immediately below each bar. Plain significantly higher than 50 wt% and 100 wt% on
day 7 (P < 0.05), no significant differences by day 14. (C) Collagen deposition on days 7 and 14. Typical
collagen staining for each condition showed immediately below each bar. 50 wt% had significantly
less collagen than 100 wt% on day 7 (P < 0.05), no significant differences by day 14. Matrix staining
is confined to the outer surfaces on plain polyHIPEs at both time points, whereas in alginate-leached
polyHIPEs, penetration into the bulk of the material is visible.
by day 14. For plain scaffolds, both ARS and to image the internal surfaces of the samples and
DR80 staining remained confined to the exterior assess cell ingrowth. The external seeding surface
scaffold surfaces at both time points. However, on is on the right side of each image.
alginate-leached scaffolds, positive staining was On the plain scaffold, cells were confined to
observed deeper within the bulk of the material. the scaffold surface, with no cell ingrowth past the
first layers of pores (Figure 6A). In the 50 wt%
3.4 Cell ingrowth is superior in porogen leached porogen leached scaffold, viable cells were visible
polyHIPE scaffolds over the internal network of the scaffold, although
In porogen leached scaffolds, cell infiltration a higher concentration of dead cells was present
is visible much further into the scaffold, with further into the structure (Figure 6B). For the
the greatest ingrowth observed in the 100 wt% 100 wt% scaffold identifying the edge of the
scaffolds (Figure 5). In plain scaffold cells are scaffold was more difficult, as there was no clear
constrained to the exterior surface and first 2 – 3 linear region due to the high macroporosity. The
rows of polyHIPE pores, achieving a maximum cells were visible deep into the scaffold internal
infiltration distance of approximately 50 μm. network and were predominately viable (green)
In contrast, in the 50 wt% scaffolds cellular cells (Figure 6C). Due to the autofluorescence
penetration had reached a depth of approximately of the polyHIPE material, there is a weak green
200 μm by day 7 and up to 450 μm by day 14. signal throughout the imaging.
For the 100 wt% scaffolds, infiltration depths over 4 Discussion
600 μm from the outer surface were observed at
both time points. In this study, we combined alginate bead porogen
3.5 Cells remain viable within the center of the leaching with emulsion templating to introduce
porogen leached polyHIPE scaffolds an additional, larger length-scale of porosity
to polyHIPEs, creating a multiscale porosity
Live/dead staining assessed by lightsheet over three length-scales. To the best of our
microscopy was performed on day 14. After knowledge, this is the first-time such multiscale
staining, the samples were cut horizontally and porous polyHIPEs have been fabricated using a
vertically and placed into the lightsheet microscope single-step alginate porogen leaching approach.
106 International Journal of Bioprinting (2020)–Volume 6, Issue 2