Page 446 - IJB-10-6
P. 446
International Journal of Bioprinting Bioprinted skin scaffolds with GNP exposure
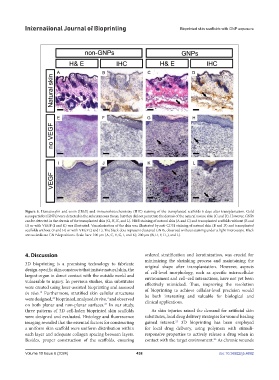
Figure 5. Hematoxylin and eosin (H&E) and immunohistochemistry (IHC) staining of the transplanted scaffolds 6 days after transplantation. Gold
nanoparticles (GNPs) were detected in the subcutaneous tissue, but they did not penetrate the dermis of the natural mouse skin (C and D). However, GNPs
can be detected in the dermis of the transplanted skin (G, H, K, and L). H&E staining of natural skin (A and C) and transplanted scaffolds without (E and
G) or with VEGF (I and K) was illustrated. Vascularization of the skin was illustrated by anti-CD31 staining of natural skin (B and D) and transplanted
scaffolds without (F and H) or with VEGF (J and L). The black dots represent clustered GNPs, observed without staining under a light microscope. Black
arrows indicate GNP depositions. Scale bars: 100 μm (A, C, E, G, I, and K); 200 μm (B, D, F, H, J, and L).
4. Discussion ordered stratification and keratinization, was crucial for
minimizing the shrinking process and maintaining the
3D bioprinting is a promising technology to fabricate original shape after transplantation. However, aspects
design-specific skin constructs that imitate natural skin, the of cell-level morphology, such as specific microcellular
largest organ in direct contact with the outside world and environment and cell–cell interactions, have not yet been
vulnerable to injury. In previous studies, skin substitutes effectively mimicked. Thus, improving the resolution
were created using laser-assisted bioprinting and assessed of bioprinting to achieve cellular-level precision would
in vivo. Furthermore, stratified skin cellular structures
12
were designed, bioprinted, analyzed in vivo, and observed be both interesting and valuable for biological and
23
2
clinical applications.
on both planar and non-planar surfaces. In our study,
24
three patterns of 3D cell-laden bioprinted skin scaffolds As skin injuries raised the demand for artificial skin
were designed and evaluated. Histology and fluorescence substitutes, local drug delivery strategies for wound healing
25
imaging revealed that the essential factors for constructing gained interest. 3D bioprinting has been employed
a uniform skin scaffold were uniform distribution within for local drug delivery, using polymers with stimuli-
each layer and adequate collagen spacing between layers. responsive properties to actively release a drug when in
Besides, proper construction of the scaffolds, ensuring contact with the target environment. As chronic wounds
26
Volume 10 Issue 6 (2024) 438 doi: 10.36922/ijb.4692