Page 221 - IJB-8-3
P. 221
Villapún, et al.
A B
C D
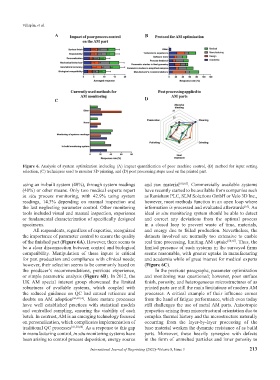
Figure 6. Analysis of system optimization including (A) impact quantification of poor machine control, (B) method for input setting
selection, (C) techniques used to monitor 3D printing, and (D) post processing steps used on the printed part.
using an in-built system (40%), through system readings and raw material [60,65] . Commercially available systems
(40%) or other means. Only two medical experts report have recently started to be available from companies such
in situ process monitoring, with 42.9% using system as Renishaw PLC, SLM Solutions GmbH or Velo 3D Inc.,
readings, 14.3% depending on manual inspection and however, most methods function in an open loop where
the last neglecting parameter control. Other monitoring information is processed and evaluated afterwards . An
[65]
tools included visual and manual inspection, experience ideal in situ monitoring system should be able to detect
or fundamental characterization of specifically designed and correct any deviations from the optimal process
specimens. in a closed loop to prevent waste of time, materials,
All respondents, regardless of expertise, recognized and energy due to failed production. Nevertheless, the
the importance of parameter control to ensure the quality datasets involved are normally too extensive to enable
of the finished part (Figure 6A). However, there seems to real time processing, limiting AM uptake [60,65] . Thus, the
be a clear disconnection between control and biological limited presence of such systems in the surveyed firms
compatibility. Manipulation of these inputs is critical seems reasonable, with greater uptake in manufacturing
for part production and compliance with clinical needs; and academia while of great interest for medical experts
however, their selection seems to be commonly based on (Figure 6C).
the producer’s recommendations, previous experience, In the previous paragraphs, parameter optimization
or simple parametric analysis (Figure 6B). In 2012, the and monitoring was questioned; however, poor surface
UK AM special interest group showcased the limited finish, porosity, and heterogeneous microstructures of as
robustness of available systems, which coupled with printed parts are still the main limitations of modern AM
the reduced guidance on QC had caused reticence and processes. A critical example of their influence comes
doubts on AM adoption [60,62,63] . More mature processes from the hand of fatigue performance, which even today
have well established practices with statistical models still challenges the use of metal AM parts. Anisotropic
and controlled sampling, ensuring the viability of each properties arising from microstructural orientation due to
batch. In contrast, AM is an emerging technology focused complex thermal history and the mesostructure naturally
on personalization, which complicates implementation of occurring from the layer-by-layer processing of the
traditional QC processes [41,50,64] . As a response to this gap base material weaken the dynamic resistance of as build
in manufacturing control, in situ monitoring systems have parts. Moreover, these heavily synergize with defects
been arising to control process deposition, energy source in the form of unmelted particles and inner porosity to
International Journal of Bioprinting (2022)–Volume 8, Issue 3 213