Page 233 - IJB-8-3
P. 233
Dong, et al.
In practical applications, a biomaterial should 3.3. Adhesive property of the hydrogel
possess sufficient fatigue resistance properties to maintain
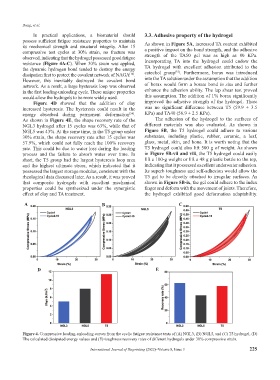
its mechanical strength and structural integrity. After 15 As shown in Figure 5A, increased TA content exhibited
compressive test cycles at 30% strain, no fracture was a positive impact on the bond strength, and the adhesive
observed, indicating that the hydrogel possessed good fatigue strength of the TA50 gel was as high as 80 KPa.
resistance (Figure 4A-C). When 30% strain was applied, Incorporating TA into the hydrogel could endow the
the dynamic hydrogen bond tended to destroy the energy TA hydrogel with excellent adhesion attributed to the
[37]
dissipation first to protect the covalent network of NAGA . catechol group . Furthermore, borax was introduced
[10]
However, this inevitably destroyed the covalent bond into the TA solution under the assumption that the addition
network. As a result, a large hysteresis loop was observed of borax would form a borate bond in situ and further
in the first loading-unloading cycle. These unique properties enhance the adhesion ability. The lap shear test proved
would allow the hydrogels to be more widely used. this assumption. The addition of 1% borax significantly
Figure 4D showed that the addition of clay improved the adhesive strength of the hydrogel. There
increased hysteresis. The hysteresis could result in the was no significant difference between T5 (59.9 ± 3.5
energy absorbed during permanent deformation . KPa) and TA40 (56.9 ± 2.5 KPa).
[34]
As shown in Figure 4E, the shape recovery rate of the The adhesion of the hydrogel to the surfaces of
NGL3 hydrogel after 15 cycles was 63%, while that of different materials was also evaluated. As shown in
NGL5 was 43%. At the same time, in the T5 group under Figure 5B, the T5 hydrogel could adhere to various
30% strain, the shape recovery rate after 15 cycles was substrates, including plastic, rubber, ceramic, a leaf,
57.9%, which could not fully reach the 100% recovery glass, metal, skin, and bone. It is worth noting that the
rate. This could be due to water loss during the loading T5 hydrogel could also lift 500 g of weight. As shown
process and the failure to absorb water over time. In in Figure 5B-vii and viii, the T5 hydrogel could easily
short, the T5 group had the largest hysteresis loop area lift a 100-g weight or lift a 48 g plastic bottle to the top,
and the highest ultimate stress, which indicated that it indicating that it possessed excellent underwater adhesion.
possessed the largest storage modulus, consistent with the Its superb toughness and self-adhesion would allow the
rheological data discussed later. As a result, it was proved T5 gel to be directly attached to irregular surfaces. As
that composite hydrogels with excellent mechanical shown in Figure 5B-ix, the gel could adhere to the index
properties could be synthesized under the synergistic finger and deform with the movement of joints. Therefore,
effect of clay and TA treatment. the hydrogel exhibited good deformation adaptability.
A B C
D E
Figure 4. Compressive loading-unloading curves from the cyclic fatigue resistance tests of (A) NGL3, (B) NGL5, and (C) T5 hydrogel. (D)
The calculated dissipated energy values and (E) toughness recovery rates of different hydrogels under 30% compressive strain.
International Journal of Bioprinting (2022)–Volume 8, Issue 3 225