Page 293 - IJB-8-4
P. 293
Fayyazbakhsh, et al.
A C
B
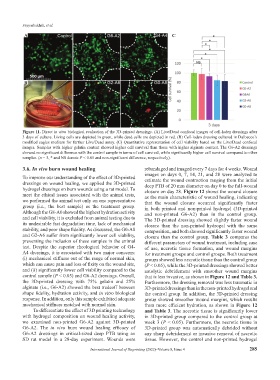
Figure 11. Direct in vitro biological evaluation of the 3D-printed dressings. (A) Live/Dead confocal images of cell-laden dressings after
3 days of culture. Living cells are depicted in green, while dead cells are depicted in red. (B) Cell-laden dressing cultured in Dulbecco’s
modified eagles medium for further Live/Dead assay. (C) Quantitative representation of cell viability based on the Live/Dead confocal
images. Samples with higher gelatin content showed higher cell survival than those with higher alginate content. The G6-A2 dressings
showed no significant difference with the control sample in terms of cell survival, while significantly higher cell survival compared to other
samples. (n = 3, * and NS denote P < 0.05 and non-significant difference, respectively).
3.6. In vivo burn wound healing rebandaged and imaged every 7 days for 4 weeks. Wound
images on days 0, 7, 14, 21, and 28 were analyzed to
To improve our understanding of the effect of 3D-printed estimate the wound contraction ranging from the initial
dressings on wound healing, we applied the 3D-printed deep PTB of 20 mm diameter on day 0 to the full-wound
hydrogel dressings on burn wounds using a rat model. To closure on day 28. Figure 12 shows the wound closure
meet the ethical issues associated with the animal tests, as the main characteristic of wound healing, indicating
we performed the animal test only on one representative that the wound closure occurred significantly faster
group (i.e., the best sample) as the treatment group. in both printed and non-printed hydrogel (3D-printed
Although the G8-A0 showed the highest hydration activity and non-printed G6-A2) than in the control group.
and cell viability, it is excluded from animal testing due to The 3D-printed dressing showed slightly faster wound
its undesirable fast degradation time, lack of mechanical closure than the non-printed hydrogel with the same
stability, and poor shape fidelity. As discussed, the G0-A8 composition, and both showed significantly faster wound
and G2-A6 suffer from significantly lower cell viability, closure than the control group. Table 3 compares the
preventing the inclusion of these samples in the animal different parameters of wound treatment, including ease
test. Despite the superior rheological behavior of G4- of use, necrotic tissue formation, and wound margins
A4 dressings, it is associated with two major concerns: for treatment groups and control groups. Both treatment
(i) mechanical stiffness out of the range of normal skin, groups showed less necrotic tissue than the control group
which can cause pain and loss of fixity on the wound site, (P < 0.05), while the 3D-printed dressings showed better
and (ii) significantly lower cell viability compared to the autolytic debridement with smoother wound margins
control sample (P < 0.05) and G6-A2 dressings. Overall, that is less invasive, as shown in Figure 12 and Table 3.
the 3D-printed dressing with 75% gelatin and 25% Furthermore, the dressing removal was less traumatic in
alginate (i.e., G6-A2) showed the best tradeoff between 3D-printed dressings than in the non-printed hydrogel and
shape fidelity, hydration activity, and in vitro biological the control group. In addition, the 3D-printed dressing
response. In addition, only this sample exhibited adequate group showed smoother wound margins, which results
mechanical stiffness matched with normal skin. from more efficient hydration, as shown in Figure 12
To differentiate the effect of 3D printing technology and Table 3. The necrotic tissue is significantly lower
with hydrogel composition on wound healing activity, in 3D-printed group compared to the control group at
we examined non-printed G6-A2 against 3D-printed week 3 (P < 0.05). Furthermore, the necrotic tissue in
G6-A2. The in vivo burn wound healing efficacy of 3D-printed group was automatically debrided without
G6-A2 dressings in critical-sized deep PTB using an any sharp debridement or invasive removal of necrotic
SD rat model in a 28-day experiment. Wounds were tissue. However, the control and non-printed hydrogel
International Journal of Bioprinting (2022)–Volume 8, Issue 4 285