Page 344 - IJB-9-2
P. 344
International Journal of Bioprinting Characterization of BITC antibacterial hydrogel
the compound hydrogels containing XG, LBG, KG, and the hand. Therefore, XLKC-Gel is more suitable for skin
CA. Compared with the hyaluronic acid carboxymethyl wound dressings attributed to its excellent extensibility
cellulose hydrogel prepared by other researchers, the and adhesion.
adhesiveness of X hydrogel is −47.5 g.s, which is higher
than that of hyaluronic acid carboxymethyl cellulose 3.3. Moisture distribution of the different hydrogels
hydrogel. The resilience is 60%, which is lower than that Low-field nuclear magnetic resonance (LF-NMR)
of hyaluronic acid carboxymethyl cellulose hydrogel . mainly reflects the law of water migration at different
[33]
Therefore, compared with hydrogels prepared by chemical states of the hydrogel using relaxation time (T2). The
crosslinking method, XLKC-Gel has lower resilience, horizontal coordinate in the water distribution map
which may be a disadvantage of hydrogels prepared by shows relaxation time, which indicates the fluidity
physical crosslinking method. However, in terms of safety of water in the hydrogel, and the vertical coordinate
of the preparation materials, XLKC-Gel prepared by shows signal intensity (proton density), which indicates
edible colloid is safer than hydrogels prepared by chemical the water content corresponding to relaxation time.
crosslinking. The transverse relaxation time of 1 – 10 ms shows T ,
2b
For skin wound repair materials, tearing wound representing strong binding of water in the gel. Similarly,
dressings applied on to especially the injuries of frequently the transverse relaxation time of 10 – 100 ms shows T ,
21
active joints can also be a potential risk for infection. representing the weak binding of water in the gel, while
Therefore, having suitable mechanical properties similar that of 100 – 1000 ms shows T , which represents easily
22
to those of skin is beneficial in maintaining the integrity flowing water in the gel, and 1000 – 10000 ms shows T ,
23
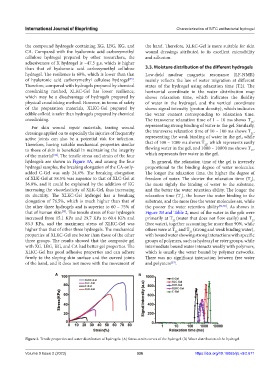
of the material . The tensile stress and strain of the four which represents free water in the gel.
[34]
hydrogels are shown in Figure 3A, and among the four In general, the relaxation time of the gel is inversely
hydrogel samples, the breaking elongation of the CA-only- proportional to the binding degree of water molecules.
added C-Gel was only 24.6%. The breaking elongation The longer the relaxation time, the higher the degree of
of XLK-Gel at 58.5% was superior to that of XLC-Gel at freedom of water. The shorter the relaxation time (T ),
2
36.8%, and it could be explained by the addition of KG the more tightly the binding of water to the substrate,
increasing the viscoelasticity of XLK-Gel, thus increasing and the better the water retention ability. The longer the
its ductility. The XLKC-Gel hydrogel has a breaking relaxation time (T ), the looser the water binding to the
2
elongation of 76.5%, which is much higher than that of substrate, and the more free the water molecules are, while
the other three hydrogels and is superior to 60 – 75% of the poorer the water retention ability [36-38] . As shown in
[35]
that of human skin . The tensile stress of four hydrogels Figure 3B and Table 2, most of the water in the gels were
increased from 15.1 KPa and 29.7 KPa to 60.4 KPa and primarily at T (water that does not flow easily) and T 23
22
83.3 KPa, and the maximum stress of XLKC-Gel was (free water), together accounting for more than 90%, while
higher than that of other three hydrogels. The mechanical others were at T and T (strong and weak binding water),
21
2b
properties of XLKC-Gel are better than those of the other with bound water showing strong interactions with specific
three groups. The results showed that the composite gel groups of polymers, such as hydroxyl or ester groups, while
with XG, LBG, KG, and CA had better gel properties. The intermediate bound water interacts weakly with polymers,
XLKC-Gel has good adhesive properties and can adhere which is usually the water bound by polymer networks.
firmly to the sloping skin surface and the curved joints There was no significant interaction between free water
of the hand, and it does not move with the movement of and polymers .
[39]
A B
Figure 3. Tensile properties and water distribution of hydrogels. (A) Stress-strain curves of the hydrogel. (B) Water distribution of the hydrogel.
Volume 9 Issue 2 (2023) 336 https://doi.org/10.18063/ijb.v9i2.671