Page 324 - IJB-9-3
P. 324
International Journal of Bioprinting New fibrillar collagen for 3D printing and bioprinting
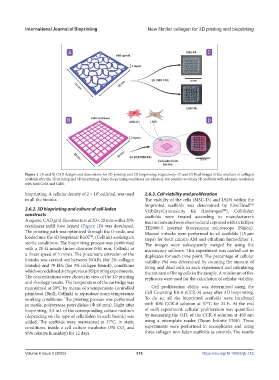
Figure 1. (A and B) CAD designs and dimensions for 3D printing and 3D bioprinting, respectively. (C and D) Real images of the resultant of collagen
scaffolds after the 3D printing and 3D bioprinting. Once the printing conditions are adjusted, it is possible to obtain 3D scaffolds with adequate resolution
with both ColA and ColN.
bioprinting. A cellular density of 2 × 10 cells/mL was used 2.6.3. Cell viability and proliferation
5
in all the bioinks. The viability of the cells (MSC-D1 and L929) within the
bioprinted scaffolds was determined by Live/Dead
TM
2.6.2. 3D bioprinting and culture of cell-laden Viability/Cytotoxicity Kit (Invitrogen ). Cell-laden
TM
constructs scaffolds were treated according to manufacturer’s
A square, CAD grid-like structure of 20 × 20 mm with a 20% instructions and were observed and captured with an Eclipse
rectilinear infill (two layers) (Figure 1B) was developed. TE2000-5 inverted fluorescence microscope (Nikon).
The printing path was optimized through the G-code, and Manual z-stacks were performed in all scaffolds (15 µm
loaded into the 3D bioprinter BioX , (Cellink) working on steps) for both calcein-AM and ethidium homodimer 1.
TM
sterile conditions. The bioprinting process was performed The images were subsequently merged by using the
with a 20-G nozzle (inner diameter 0.61 mm, Cellink) at microscopy software. This experiment was carried out in
a linear speed of 5 mm/s. The pneumatic extrusion of the duplicates for each time point. The percentage of cellular
bioinks was carried out between 50 kPa (for 2% collagen viability (%) was determined by counting the amount of
bioinks) and 70 kPa (for 3% collagen bioink), conditions living and dead cells in each experiment and calculating
which were defined in the previous 3D printing experiments. the amount of living cells in the sample. A minimum of five
The concentrations were chosen in view of the 3D printing replicates were used for the calculation of cellular viability.
and rheology results. The temperature of the cartridge was
maintained at 20°C by means of a temperature-controlled Cell proliferation ability was determined using the
printhead (BioX, Cellink) to reproduce room temperature Cell Counting Kit-8 (CCK-8) assay after 3D bioprinting.
working conditions. The printing process was performed To do so, all the bioprinted scaffolds were incubated
in sterile, polystyrene petri dishes (Ф 60 mm). Right after with 10% CCK-8 solution at 37°C for 24 h. At the end
bioprinting, 3.5 mL of the corresponding culture medium of each experiment, cellular proliferation was quantified
(depending on the type of cells laden in each bioink) was by measuring the O.D. of the CCK-8 solution at 450 nm
added. The scaffolds were maintained at 37°C, in static using a microplate reader (Tecan Infinite F200). These
conditions, inside a cell culture incubator (5% CO and experiments were performed in sextuplicates and using
2
95% relative humidity) for 12 days. three collagen non-laden scaffolds as controls. The results
Volume 9 Issue 3 (2023) 316 https://doi.org/10.18063/ijb.712