Page 355 - IJB-9-3
P. 355
International Journal of Bioprinting Decellularized materials for bioprinting of liver constructs
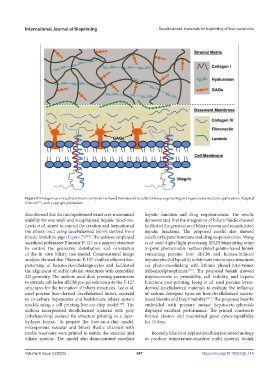
Figure 5. Endogenous extracellular matrix components-based biomaterials suitable for tissue engineering and regenerative medicine applications. Adapted
[97]
from ref. , with copyright permission.
data showed that the micropatterned structures-maintained hepatic function and drug responsiveness. The results
viability for one week and recapitulated hepatic functions. demonstrated that the integration of biliary fluidic channel
Lewis et al. aimed to control the creation and formation of facilitated the generation of biliary system and recapitulated
the biliary tract using decellularized bioink derived from hepatic functions. The proposed model also showed
female Yorkshire pigs (Figure 7) [128] . The authors employed excellent hepatic functions and drug responsiveness. Wang
sacrificial poloxamer Pluronic F-127 as a support structure et al. used digital light processing (DLP) bioprinting setup
to control the geometric distribution and orientation to print photocurable methacrylated gelatin-based bioink
of the in vitro biliary tree model. Computational image containing porcine liver dECM and human-induced
analysis showed that Pluronic F-127 enabled efficient bio- hepatocytes (hiHep cells) to fabricate microtissue structures
patterning of hepatocytes/cholangiocytes and facilitated via photo-crosslinking with lithium phenyl-2,4,6-trimet
the alignment of stable tubular structures with controlled hylbenzoylphosphinate [130] . The proposed bioink showed
2D geometry. The authors used dual printing parameters improvements in printability, cell viability, and hepatic
to extrude cell-laden dECM pre-gel solution into the F-127 functions post-printing. Jeong et al. used porcine livers-
structures for the formation of biliary structures. Lee et al. derived decellularized materials to evaluate the influence
used porcine liver-derived decellularized bioink material of various detergent types on liver-decellularized matrix-
to co-culture hepatocytes and biofabricate biliary system based bioinks and bioprintability [131] . The proposed bioinks
models using a cell printing/liver-on-chip model [129] . The embedded with primary mouse hepatocyte-spheroids
authors incorporated decellularized material with poly displayed excellent performance. The printed constructs
(ethylene/vinyl acetate) for structure printing in a layer- formed clusters and maintained good cytocompatibility
by-layer format. To prepare the liver-on-a-chip model, for 14 days.
microporous vascular and biliary fluidic channels with
media reservoirs were printed to mimic the vascular and Recently, Khati et al. applied decellularization technology
biliary systems. The model also demonstrated excellent to produce temperature-sensitive multi-material bioink
Volume 9 Issue 3 (2023) 347 https://doi.org/10.18063/ijb.714